Atoms are the building blocks of matter, and understanding their structure is fundamental to chemistry and physics. While protons define an element and electrons govern its reactivity, neutrons play a crucial role in stability and isotopic variation. Knowing how to calculate the number of neutrons in an atom is a basic but essential skill for students, educators, and science enthusiasts. This guide breaks down the process clearly and accurately, using accessible language and practical tools.
Understanding Atomic Structure Basics
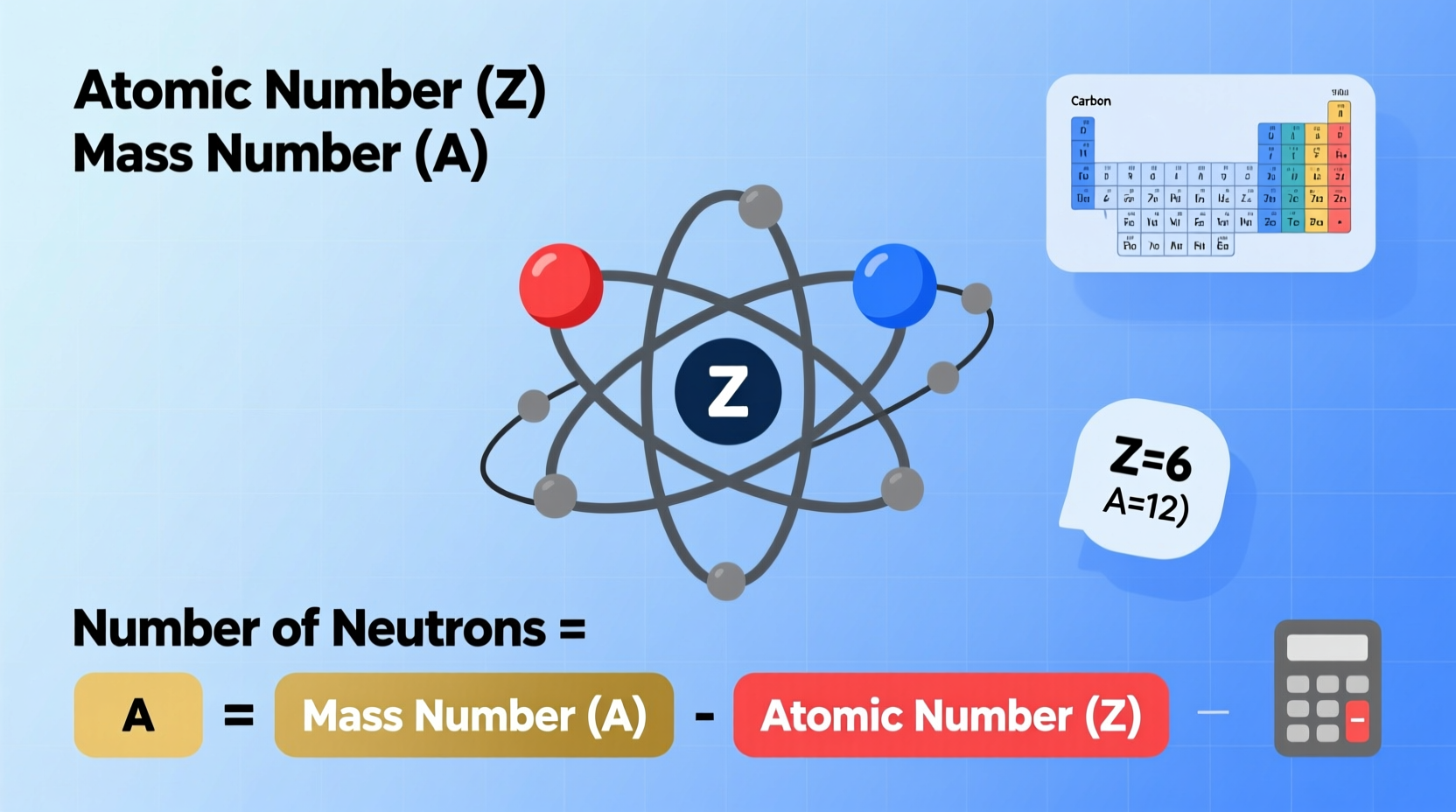
Every atom consists of three primary subatomic particles: protons, neutrons, and electrons. Protons carry a positive charge and reside in the nucleus. Electrons, negatively charged, orbit the nucleus. Neutrons, as their name suggests, are neutral—they have no charge—and are also located in the nucleus.
The identity of an element is determined by its number of protons, known as the atomic number (Z). For example, every carbon atom has 6 protons, so its atomic number is 6. The mass number (A), on the other hand, is the total number of protons and neutrons in the nucleus. Since electrons contribute negligibly to atomic mass, they are not included in this sum.
With these two values—atomic number and mass number—you can determine the number of neutrons using a straightforward formula:
How to Calculate Neutrons: The Core Formula
The number of neutrons in an atom is calculated by subtracting the atomic number from the mass number:
Number of Neutrons = Mass Number (A) – Atomic Number (Z)
This formula works for any isotope of any element. Let’s walk through what each component means:
- Mass Number (A): Total number of protons + neutrons in a specific atom (always a whole number).
- Atomic Number (Z): Number of protons in the nucleus (unique to each element).
For example, consider an atom of sodium-23. The “23” is the mass number. Sodium’s atomic number is 11 (from the periodic table). Applying the formula:
Neutrons = 23 – 11 = 12
So, a sodium-23 atom has 12 neutrons.
“Understanding neutron count helps predict nuclear stability and behavior in reactions.” — Dr. Lena Patel, Nuclear Chemist
Step-by-Step Guide to Finding Neutron Count
Follow this clear sequence to determine the number of neutrons in any atom:
- Identify the element (e.g., oxygen, iron, uranium).
- Find the atomic number (Z) on the periodic table. This is the number of protons.
- Determine the mass number (A):
- If an isotope is specified (e.g., carbon-14), the number after the hyphen is the mass number.
- If no isotope is given, use the atomic weight from the periodic table and round it to the nearest whole number.
- Subtract the atomic number from the mass number.
- Verify your result: ensure the neutron count is non-negative and reasonable for the element.
This method applies universally, whether you're analyzing hydrogen or plutonium.
Real Example: Calculating Neutrons in Chlorine Isotopes
Chlorine exists naturally as a mixture of two stable isotopes: chlorine-35 and chlorine-37. Both have the same atomic number (17), but different mass numbers.
For chlorine-35:
Neutrons = 35 – 17 = 18
For chlorine-37:
Neutrons = 37 – 17 = 20
Despite having different neutron counts, both are chlorine because they each have 17 protons. This variation explains why chlorine’s atomic weight on the periodic table is approximately 35.45—it’s a weighted average of both isotopes’ masses based on abundance.
This case illustrates why knowing the specific isotope matters. Without that detail, you can only estimate the most common neutron count.
Common Pitfalls and How to Avoid Them
Missteps in neutron calculations often stem from confusion between atomic weight and mass number. Here’s a comparison to clarify:
| Term | Definition | Example (Carbon) |
|---|---|---|
| Atomic Number (Z) | Number of protons | 6 |
| Mass Number (A) | Protons + Neutrons (specific isotope) | 12 (carbon-12) |
| Atomic Weight | Average mass of all isotopes (decimal) | 12.01 |
One frequent error is using the atomic weight directly without rounding. For instance, using 12.01 instead of 12 for carbon leads to a fractional neutron count, which is impossible. Always round the atomic weight to the nearest whole number when a specific isotope isn’t provided.
Quick Reference Checklist
Use this checklist whenever you need to calculate neutron count:
- ☑ Identify the element and locate its atomic number (Z) on the periodic table.
- ☑ Determine the mass number (A)—either from isotope notation or by rounding the atomic weight.
- ☑ Apply the formula: Neutrons = A – Z.
- ☑ Double-check that the result is a positive whole number.
- ☑ Confirm consistency: elements in the same isotopic series vary only in neutrons.
Frequently Asked Questions
Can an atom have zero neutrons?
Yes, but only in one case: the most common isotope of hydrogen, protium (hydrogen-1), has one proton and zero neutrons. It’s the only stable atom without neutrons.
Why do isotopes have different numbers of neutrons?
Isotopes are variations of the same element with differing neutron counts. This affects atomic mass and nuclear stability but not chemical properties, which are governed by electron configuration.
Do neutrons affect an element’s reactivity?
Not directly. Neutrons influence nuclear properties like radioactivity and stability, but chemical behavior is determined by the number and arrangement of electrons, which equals the number of protons in a neutral atom.
Mastering the Basics Builds Scientific Confidence
Calculating the number of neutrons is more than a classroom exercise—it’s a gateway to deeper understanding of atomic behavior, radioactivity, and material science. Whether you're preparing for an exam, teaching a lesson, or simply curious about the world at the atomic level, mastering this simple calculation empowers you to explore further. From predicting isotope stability to interpreting nuclear reactions, this foundational knowledge opens doors.
Remember, precision starts with clarity. Use the periodic table wisely, distinguish between mass number and atomic weight, and always verify your inputs. With practice, this process becomes second nature.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?