Understanding whether a substance is polar or nonpolar is essential in chemistry, biology, and even everyday applications like cleaning, cooking, and pharmaceuticals. Polarity determines how molecules interact—with water, with each other, and with biological systems. While the concept may seem complex at first, identifying polar and nonpolar substances becomes straightforward once you know the key principles. This guide breaks down the process into clear, actionable steps anyone can follow.
Step 1: Understand What Makes a Molecule Polar
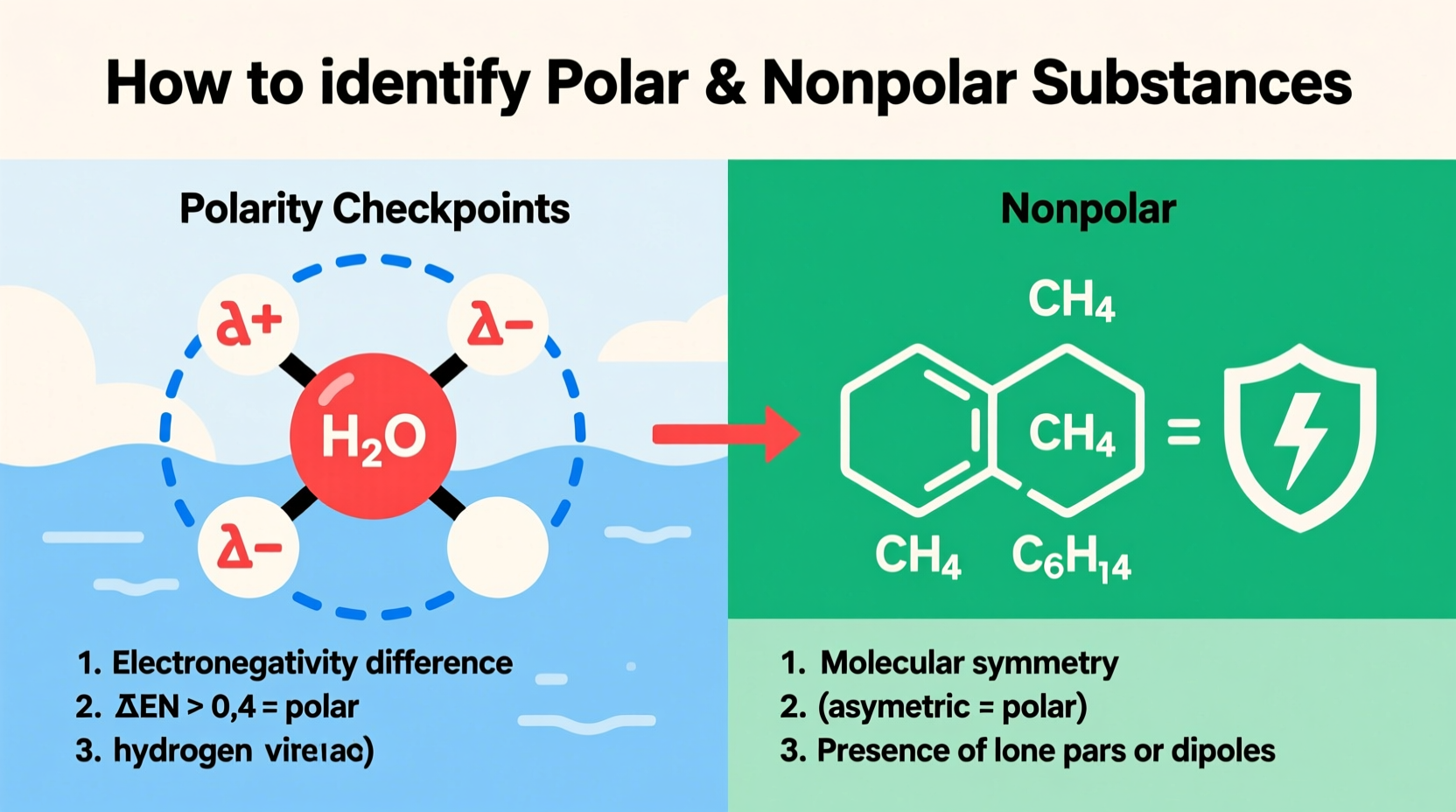
Polarity arises from an uneven distribution of electrons in a molecule. This happens when atoms with different electronegativities form covalent bonds. Electronegativity is a measure of how strongly an atom attracts shared electrons. When two bonded atoms have significantly different electronegativity values, the more electronegative atom pulls electron density toward itself, creating a dipole—a partial negative charge (δ⁻) on one end and a partial positive charge (δ⁺) on the other.
A molecule is considered polar if:
- It contains polar bonds (due to electronegativity differences), AND
- The dipoles do not cancel out due to molecular geometry.
In contrast, nonpolar molecules either have no polar bonds or possess symmetrical shapes that cause bond dipoles to cancel out.
Step 2: Identify Electronegativity Differences
To determine if a bond is polar, compare the electronegativity values of the atoms involved. Linus Pauling’s electronegativity scale is commonly used. Here are general guidelines:
| Electronegativity Difference | Bond Type |
|---|---|
| 0.0 – 0.4 | Nonpolar covalent |
| 0.5 – 1.6 | Polar covalent |
| > 1.7 | Ionic (but still relevant for polarity context) |
For example:
- C–H bond: ΔEN ≈ 0.4 → considered nonpolar covalent
- O–H bond: ΔEN ≈ 1.4 → highly polar covalent
- C–O bond: ΔEN ≈ 1.0 → polar covalent
If all bonds in a molecule are nonpolar, the molecule itself is likely nonpolar. But if there are polar bonds, proceed to geometry analysis.
Step 3: Analyze Molecular Geometry
Even with polar bonds, a molecule can be nonpolar if its shape allows dipoles to cancel. This is where VSEPR (Valence Shell Electron Pair Repulsion) theory comes into play. Common geometries include linear, trigonal planar, tetrahedral, bent, and trigonal pyramidal.
Consider these examples:
- CO₂: Linear molecule with two polar C=O bonds. Because the dipoles point in opposite directions and are equal in magnitude, they cancel. Result: nonpolar.
- H₂O: Bent shape due to lone pairs on oxygen. The O–H dipoles do not cancel. Result: polar.
- CH₄: Tetrahedral symmetry. All C–H bonds are identical and symmetrically arranged. Dipoles cancel. Result: nonpolar.
- NH₃: Trigonal pyramidal. Lone pair creates asymmetry. N–H dipoles don’t cancel. Result: polar.
“Molecular symmetry is just as important as bond polarity when determining overall polarity.” — Dr. Rebecca Langston, Physical Chemist, University of Colorado
Step 4: Apply the Step-by-Step Identification Method
Follow this systematic checklist to confidently classify any substance:
- Determine the type of bonds: Are there electronegativity differences greater than 0.4? If not, the molecule is likely nonpolar.
- Draw the Lewis structure: Identify bonding patterns and lone pairs on the central atom.
- Predict molecular geometry: Use VSEPR theory based on electron domains (bonds + lone pairs).
- Check for symmetry: Are polar bonds arranged symmetrically? If yes, dipoles may cancel.
- Evaluate net dipole moment: If the vector sum of bond dipoles is zero → nonpolar. If not → polar.
Step 5: Real-World Application – A Mini Case Study
Sarah, a college student preparing for her organic chemistry lab, needed to separate two liquids: ethanol and hexane. She knew ethanol dissolves in water but hexane does not. To understand why, she applied polarity principles.
She started with ethanol (C₂H₅OH). It has a hydroxyl (–OH) group with a highly polar O–H bond. The molecule is bent near oxygen, preventing dipole cancellation. Conclusion: polar—and therefore miscible with water.
Next, hexane (C₆H₁₄). Only C–C and C–H bonds, both with negligible electronegativity differences. Symmetrical chain structure. No net dipole. Conclusion: nonpolar—immiscible with water, forms a separate layer.
This understanding helped Sarah design an effective liquid-liquid extraction, using water to isolate the polar component from the mixture.
Quick Reference Checklist: Is Your Substance Polar?
Use this checklist for fast assessment:
- ✅ Does it contain O–H, N–H, or F–H bonds? → Likely polar
- ✅ Is there a significant electronegativity difference between bonded atoms? → Check further
- ✅ Is the molecule asymmetric (e.g., bent, pyramidal)? → Likely polar
- ❌ Are polar bonds arranged symmetrically (e.g., CO₂, CCl₄)? → Nonpolar
- ❌ Does it consist only of C–C and C–H bonds? → Typically nonpolar
Frequently Asked Questions
Can a molecule with polar bonds be nonpolar?
Yes. Carbon tetrachloride (CCl₄) is a classic example. Each C–Cl bond is polar, but the tetrahedral symmetry causes the four dipoles to cancel out, resulting in a nonpolar molecule despite having polar bonds.
Is oil polar or nonpolar?
Oil is nonpolar. Most oils are long hydrocarbon chains (like triglycerides) composed of C–C and C–H bonds with minimal electronegativity differences. This is why oil doesn’t mix with water—a polar solvent.
Does polarity affect boiling point?
Yes. Polar substances generally have higher boiling points than nonpolar ones of similar size because they experience stronger intermolecular forces, such as dipole-dipole interactions and hydrogen bonding.
Final Tips for Mastering Polarity Identification
Practice makes perfect. Start with simple molecules like H₂O, CO₂, NH₃, and CH₄. Sketch their structures, assess bond polarity, and evaluate geometry. Over time, you’ll develop intuition for recognizing patterns.
Keep in mind exceptions and nuances. For instance, cis- and trans- isomers of disubstituted alkenes can differ in polarity due to spatial arrangement—even with identical atoms. The cis form is usually polar; the trans form may be nonpolar due to symmetry.
Also, consider context. In biological systems, polarity determines membrane permeability. Drugs must balance polarity to cross lipid bilayers yet remain soluble in blood (aqueous environment). Understanding polarity helps explain drug delivery, solubility, and reactivity.
Conclusion: Put Knowledge Into Practice
Identifying polar and nonpolar substances doesn’t require advanced tools—just a logical approach grounded in electronegativity and molecular shape. By following the steps outlined here, you can confidently analyze any molecule and predict its behavior in solutions, reactions, and real-life scenarios. Whether you're a student, educator, or science enthusiast, mastering this skill opens doors to deeper understanding across chemistry and related fields.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?