DNA replication is a fundamental biological process that ensures genetic information is accurately passed from one generation of cells to the next. Central to this process are two newly synthesized DNA strands: the leading strand and the lagging strand. While both are essential, they are formed in strikingly different ways. The reason the lagging strand exists—and why it must be synthesized discontinuously—lies in the biochemical properties of DNA polymerase and the antiparallel nature of the DNA double helix.
Understanding the distinction between these two strands is crucial for grasping how life maintains genetic fidelity at the molecular level. This article explores the mechanisms behind leading and lagging strand synthesis, explains why the lagging strand occurs, and highlights the cellular machinery that makes it all possible.
The Antiparallel Nature of DNA
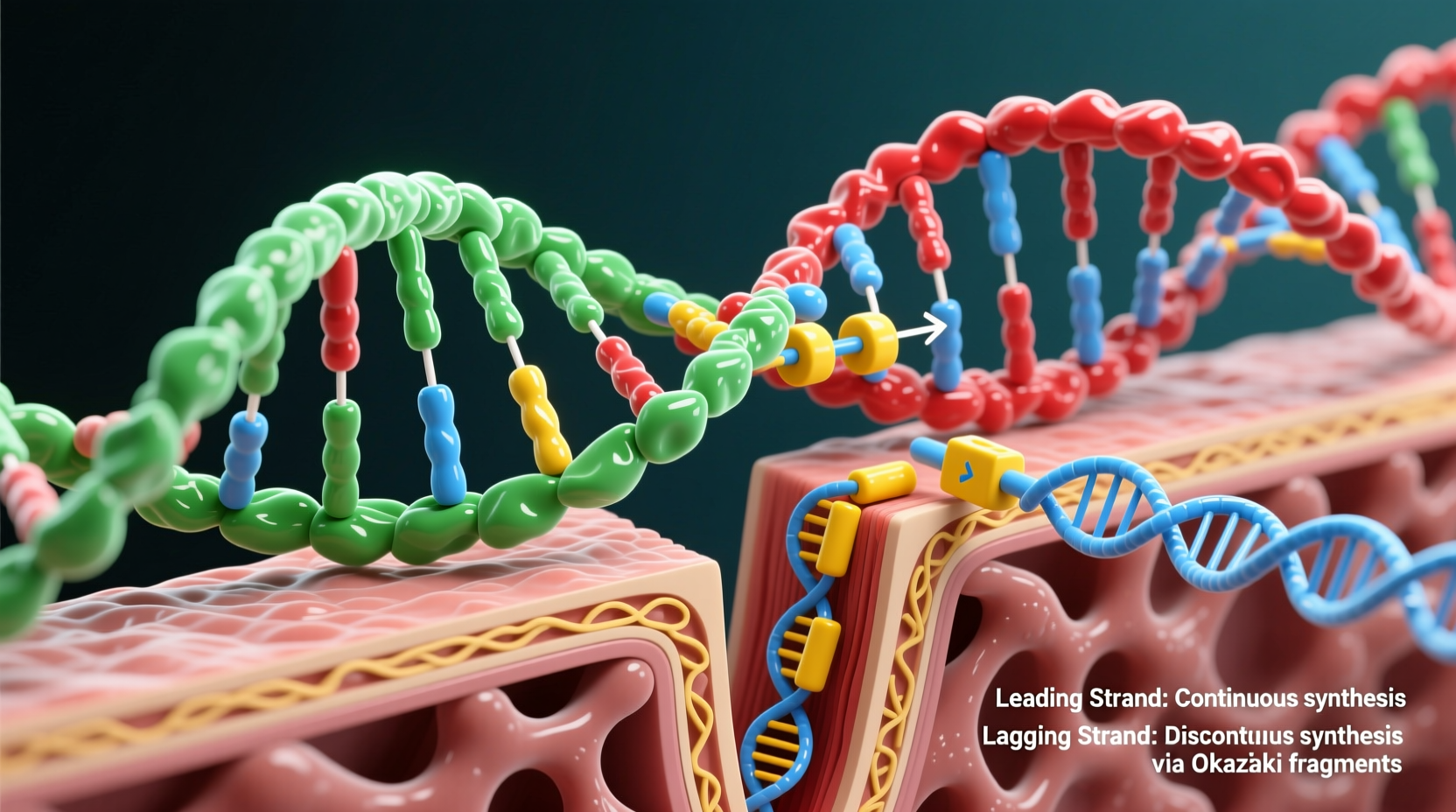
DNA is composed of two complementary strands that run in opposite directions—one in the 5' to 3' direction and the other in the 3' to 5'. This structural feature is known as antiparallel orientation. During replication, the double helix unwinds, and each strand serves as a template for a new complementary strand.
The enzyme responsible for synthesizing new DNA, DNA polymerase, can only add nucleotides in one direction: from the 5' end to the 3' end. This means it can only extend a growing DNA chain by attaching new nucleotides to the free 3' hydroxyl group. This unidirectional activity creates a fundamental asymmetry during replication.
Because the two template strands are oriented in opposite directions, DNA polymerase can continuously synthesize one new strand (the leading strand) but must work in fragments on the other (the lagging strand). This constraint is the root cause of the lagging strand phenomenon.
Leading Strand: Continuous Synthesis
The leading strand is synthesized continuously in the same direction as the replication fork moves. As the helicase enzyme unwinds the DNA ahead, the polymerase follows closely behind, adding nucleotides smoothly along the 3' to 5' template strand. Because the polymerase's preferred direction (5' → 3') aligns with the movement of the replication fork, no interruptions occur.
This continuous synthesis is efficient and straightforward. Only one RNA primer is needed at the origin of replication, after which DNA polymerase III (in prokaryotes) or polymerase δ/ε (in eukaryotes) extends the strand without pause.
Lagging Strand: Discontinuous Fragmentation
The lagging strand is built on the template that runs 5' to 3', forcing DNA polymerase to work in the opposite direction of the replication fork’s progression. Since the enzyme cannot synthesize DNA in the 3' → 5' direction, it must repeatedly start and stop, creating short segments known as Okazaki fragments.
Each fragment begins with an RNA primer laid down by primase. DNA polymerase then extends the primer in the 5' → 3' direction until it reaches the previous fragment. These fragments typically range from 1,000–2,000 nucleotides long in prokaryotes and 100–200 in eukaryotes, reflecting differences in replication complexity.
After synthesis, RNA primers are removed by enzymes like RNase H or DNA polymerase I (in bacteria), and the gaps are filled with DNA. Finally, DNA ligase seals the nicks between adjacent fragments, forming a continuous strand.
“Without the ability to create Okazaki fragments, the cell would be unable to replicate the entire genome. The lagging strand is not a flaw—it’s a necessary adaptation to enzymatic constraints.” — Dr. Linda Chen, Molecular Biologist, University of California, Berkeley
Key Enzymes Involved in Lagging Strand Synthesis
The discontinuous nature of the lagging strand demands a more complex set of proteins than the leading strand. Several key enzymes coordinate to ensure accurate and efficient replication:
- Primase: Synthesizes short RNA primers to initiate each Okazaki fragment.
- DNA Polymerase III (prokaryotes) / Pol δ (eukaryotes): Extends the RNA primer with DNA nucleotides.
- RNase H and FEN1: Remove RNA primers after extension.
- DNA Polymerase I (bacteria): Replaces RNA with DNA in the gap left behind.
- DNA Ligase: Joins the sugar-phosphate backbones of adjacent fragments.
- Single-Strand Binding Proteins (SSBs): Stabilize the exposed single-stranded template.
- Clamp Loader and Sliding Clamp (PCNA in eukaryotes): Keep polymerase attached to the template for efficiency.
This intricate coordination ensures that despite its fragmented synthesis, the lagging strand is completed with high fidelity.
Step-by-Step: How the Lagging Strand Is Formed
The formation of the lagging strand follows a precise sequence of events:
- Unwinding: Helicase separates the DNA double helix, creating a replication fork.
- Primer Synthesis: Primase adds a short RNA primer (~10 nucleotides) to the lagging strand template.
- Fragment Extension: DNA polymerase begins synthesizing an Okazaki fragment in the 5' → 3' direction, moving away from the fork.
- Fork Progression: As the fork advances, another primer is laid further downstream, initiating the next fragment.
- Primer Removal: Once the fragment is complete, RNA primers are excised by nucleases.
- Gap Filling: DNA polymerase fills the resulting gap with complementary DNA.
- Ligation: DNA ligase joins the fragments into a continuous strand.
This cycle repeats hundreds or thousands of times per replication event, depending on the organism and chromosome size.
Comparison: Leading vs Lagging Strand
| Feature | Leading Strand | Lagging Strand |
|---|---|---|
| Synthesis Direction | Continuous, same direction as fork movement | Discontinuous, opposite to fork movement |
| Primer Requirement | One initial RNA primer | Multiple RNA primers (one per fragment) |
| Okazaki Fragments | None | Present (100–2000 nt long) |
| Processing Complexity | Low | High (primer removal, ligation) |
| Main Enzymes Involved | DNA Pol III, helicase, SSBs | Primase, Pol III/I, RNase H, ligase |
Why Doesn’t Evolution Eliminate the Lagging Strand?
A common question arises: if the lagging strand introduces complexity, errors, and energy costs, why hasn’t evolution selected for a simpler mechanism? The answer lies in biochemistry itself—DNA polymerase’s strict 5' → 3' synthesis direction is conserved across all domains of life because it enables proofreading.
Polymerases have exonuclease activity that allows them to detect and remove mismatched nucleotides. This function only works when synthesis proceeds 5' → 3', making the directional constraint a trade-off for accuracy. The cost of discontinuous synthesis is far less than the risk of unchecked mutations.
In essence, the lagging strand is not inefficient—it’s a necessary compromise that prioritizes genomic stability over simplicity.
Mini Case Study: Mutation in DNA Ligase
In a clinical case study, a patient presented with increased sensitivity to UV radiation and a predisposition to skin cancer. Genetic sequencing revealed a mutation in the gene encoding DNA ligase I. This enzyme, critical for joining Okazaki fragments, was partially nonfunctional.
As a result, the patient’s cells accumulated nicks in the lagging strand during replication. These unresolved breaks led to DNA fragmentation under stress, impairing cell division and increasing genomic instability. This real-world example underscores the importance of every step in lagging strand maturation—even a single defective enzyme can have systemic consequences.
FAQ
Why can’t DNA polymerase synthesize in the 3' to 5' direction?
DNA polymerase relies on a free 3' hydroxyl group to form a phosphodiester bond with the incoming nucleotide. Without this reactive group at the 5' end, elongation in the reverse direction is chemically impossible. Additionally, 5' → 3' synthesis allows for proofreading via 3' → 5' exonuclease activity, enhancing fidelity.
Are Okazaki fragments found in both prokaryotes and eukaryotes?
Yes, Okazaki fragments occur in both domains. However, they are shorter in eukaryotes (100–200 nucleotides) due to nucleosome spacing and chromatin structure, compared to 1,000–2,000 in prokaryotes.
Is the lagging strand more prone to errors?
While the lagging strand undergoes more processing steps, error rates remain low due to robust repair mechanisms. However, some studies suggest slightly higher mutation rates in lagging strand templates, possibly due to exposure of single-stranded DNA or timing of repair pathways.
Checklist: Key Concepts to Remember
- ✔ DNA polymerase only synthesizes DNA in the 5' → 3' direction.
- ✔ The antiparallel structure of DNA necessitates two different synthesis mechanisms.
- ✔ The leading strand is continuous; the lagging strand is made in fragments.
- ✔ Okazaki fragments require RNA primers, which are later replaced and sealed.
- ✔ Multiple enzymes—including primase, ligase, and nucleases—are essential for lagging strand completion.
- ✔ The lagging strand is not a flaw but an evolutionary solution to biochemical constraints.
Conclusion
The existence of the lagging strand is not arbitrary—it is a direct consequence of the physical and chemical rules governing DNA replication. While the leading strand progresses smoothly, the lagging strand exemplifies cellular ingenuity in overcoming directional limitations through segmented synthesis. Far from being a drawback, this mechanism ensures that genetic information is copied with remarkable precision across billions of cell divisions.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?