The acid dissociation constant, or pKa, is a fundamental concept in chemistry that quantifies the strength of an acid in solution. It plays a crucial role in pharmaceutical development, environmental science, biochemistry, and organic synthesis. Despite its importance, many students and professionals struggle with accurately determining pKa values due to the variety of methods available and the subtleties involved in experimental conditions. This guide provides a clear, systematic approach to finding pKa values—whether through calculation, measurement, or estimation—with practical tips and real-world context.
Understanding pKa: The Foundation
pKa is defined as the negative logarithm (base 10) of the acid dissociation constant (Ka):
pKa = –log₁₀(Ka)
A lower pKa value indicates a stronger acid—meaning it donates protons more readily. Conversely, a higher pKa suggests a weaker acid. For example, hydrochloric acid (HCl) has a pKa of approximately –7, making it very strong, while acetic acid has a pKa of 4.76, classifying it as a weak acid.
Understanding pKa helps predict reaction outcomes, solubility, drug absorption, and buffer behavior. In biological systems, knowing the pKa of amino acid side chains is essential for understanding protein folding and enzyme activity.
Step-by-Step Guide to Finding pKa Experimentally
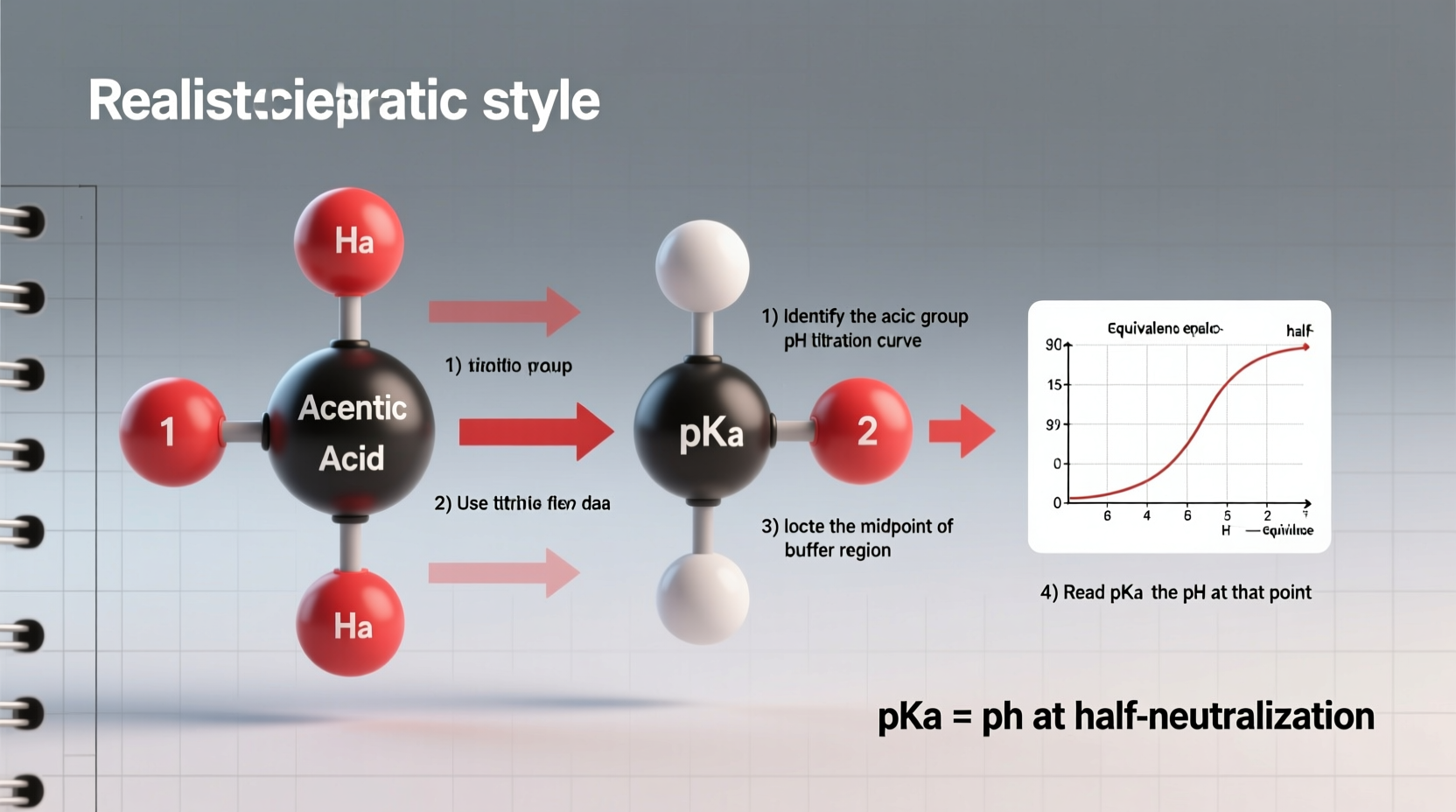
When precision is required, experimental determination remains the gold standard. Here’s a proven method using potentiometric titration:
- Prepare your acid solution: Dissolve a known amount of the acid in water or a suitable solvent. Ensure purity and accurate concentration.
- Set up a pH meter and burette: Calibrate the pH meter using standard buffer solutions (e.g., pH 4.00, 7.00).
- Titrating with base: Slowly add a standardized solution of sodium hydroxide (NaOH) while continuously monitoring the pH.
- Record data points: Note the volume of base added and corresponding pH after each increment, especially near the equivalence point.
- Plot the titration curve: Graph pH (y-axis) versus volume of base (x-axis). Identify the half-equivalence point—the midpoint of the buffering region.
- Determine pKa: At the half-equivalence point, pH = pKa. Read the pH value directly from the graph at this volume.
This method works best for monoprotic acids. For polyprotic acids (e.g., phosphoric acid), multiple pKa values can be determined from successive buffering regions.
Estimating pKa Using Structure-Based Rules
When laboratory access is limited, chemists often rely on structure-activity relationships to estimate pKa. Functional groups have characteristic ranges:
| Functional Group | Typical pKa Range | Example Compound |
|---|---|---|
| Carboxylic acid | 3–5 | Acetic acid (4.76) |
| Phenol | 9–10 | Phenol (9.99) |
| Alcohol | 15–18 | Ethanol (~15.9) |
| Ammonium ion (R-NH₃⁺) | 9–11 | Methylammonium ion (10.6) |
| Sulfonamide | 8–10 | Acetazolamide (~9.0) |
Electron-withdrawing groups (e.g., –NO₂, –F) near the acidic site lower pKa by stabilizing the conjugate base. Resonance effects, such as in carboxylates, also enhance acidity. Use these trends to make informed predictions before running experiments.
“Structure-based pKa estimation isn’t perfect, but it gives you a starting point that can save hours in the lab.” — Dr. Lena Torres, Medicinal Chemist, University of Colorado
Using Computational Tools and Databases
Modern computational chemistry offers powerful alternatives for predicting pKa. Software tools like MarvinSketch, ACD/pKa DB, and SPARC calculate pKa based on molecular structure using algorithms trained on experimental data.
Here’s how to use them effectively:
- Draw or input the molecular structure into the software.
- Select the prediction mode (aqueous, microspecies, macroconstants).
- Review predicted pKa values for all ionizable sites.
- Cross-reference with literature if possible.
These tools are particularly valuable in drug discovery, where thousands of compounds must be screened rapidly. However, they may struggle with exotic structures or tautomeric systems, so always validate critical results experimentally.
Mini Case Study: Optimizing Drug Solubility
A pharmaceutical research team was developing a new antiviral compound with poor solubility at physiological pH. Initial tests showed low oral bioavailability. By analyzing the molecule, they identified a pyridine nitrogen with a predicted pKa of 5.2. At stomach pH (~1.5), the nitrogen would be protonated, enhancing solubility. But in the intestines (pH ~6.5), it would deprotonate and precipitate.
Using both computational modeling and titration, they confirmed the pKa and redesigned the molecule by adding an electron-withdrawing fluorine atom adjacent to the nitrogen. This lowered the pKa to 3.8, ensuring the compound remained uncharged and soluble throughout the digestive tract. The revised version showed a 40% increase in absorption in preclinical trials.
This case illustrates how precise pKa knowledge directly impacts real-world outcomes in medicine.
Common Pitfalls and How to Avoid Them
Even experienced researchers can misjudge pKa under certain conditions. Watch out for these common errors:
- Ignoring solvent effects: pKa in water differs significantly from that in DMSO or ethanol. Never assume transferability.
- Poor calibration: An uncalibrated pH meter can introduce errors of ±0.3 units or more—significant when pKa differences drive reactivity.
- Overlooking ionic strength: High salt concentrations shift pKa via the Debye-Hückel effect. Report ionic conditions alongside results.
- Misidentifying the half-equivalence point: In asymmetric titration curves, visual inspection can mislead. Use derivative plots (dpH/dV) to locate inflection points accurately.
Checklist: Ensuring Accuracy When Determining pKa
Follow this checklist to ensure reliable results every time:
- ✅ Use high-purity chemicals and deionized water.
- ✅ Calibrate the pH meter with at least two standard buffers.
- ✅ Record temperature during measurement (pKa is temperature-dependent).
- ✅ Stir solution gently but consistently during titration.
- ✅ Take frequent pH readings near expected equivalence points.
- ✅ Confirm results with duplicate runs.
- ✅ Compare findings with literature or computational models.
Frequently Asked Questions
Can I find pKa without a lab setup?
Yes. You can use online databases like PubChem, DrugBank, or ChemSpider to look up experimentally measured pKa values. If unavailable, predictive software such as Epik (Schrödinger) or ChemAxon can provide estimates based on molecular structure.
Why does pKa change with temperature?
Acid dissociation is an equilibrium process influenced by thermodynamics. As temperature increases, the equilibrium constant (Ka) shifts, altering pKa. For example, water’s pKa decreases from 14.0 at 25°C to 12.6 at 100°C, meaning it becomes a stronger acid at higher temperatures.
Is pKa the same as pH?
No. pH measures the acidity of a solution (i.e., [H⁺] concentration), while pKa is a property of a specific acid indicating its tendency to donate a proton. However, when pH = pKa, the acid and its conjugate base are present in equal concentrations—a key principle in buffer design.
Final Thoughts and Action Steps
Mastering how to find the pKa is not just about following procedures—it's about understanding the chemical principles behind acid-base behavior and applying them with precision. Whether you're optimizing a drug candidate, designing a catalyst, or teaching foundational chemistry, accurate pKa determination enhances decision-making and improves outcomes.
Start by practicing titrations with well-known acids like acetic or benzoic acid to build confidence. Then, expand to unknowns and explore computational tools to complement your hands-on skills. Keep detailed records, question discrepancies, and always consider the broader context—solvent, temperature, and molecular environment matter.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?