In molecular biology, one of the most fundamental distinctions between DNA replication and transcription lies in the requirement for a primer. While DNA polymerase—the enzyme responsible for copying DNA during replication—cannot begin synthesis without a short RNA or DNA primer, RNA polymerase operates differently. It initiates RNA synthesis de novo, meaning from scratch, without needing any pre-existing strand to extend. This capability is not just a minor biochemical nuance; it's central to how gene expression begins in all living organisms. Understanding why RNA polymerase doesn’t require a primer reveals deep insights into cellular efficiency, regulation, and evolutionary design.
How RNA Polymerase Initiates Transcription Without a Primer
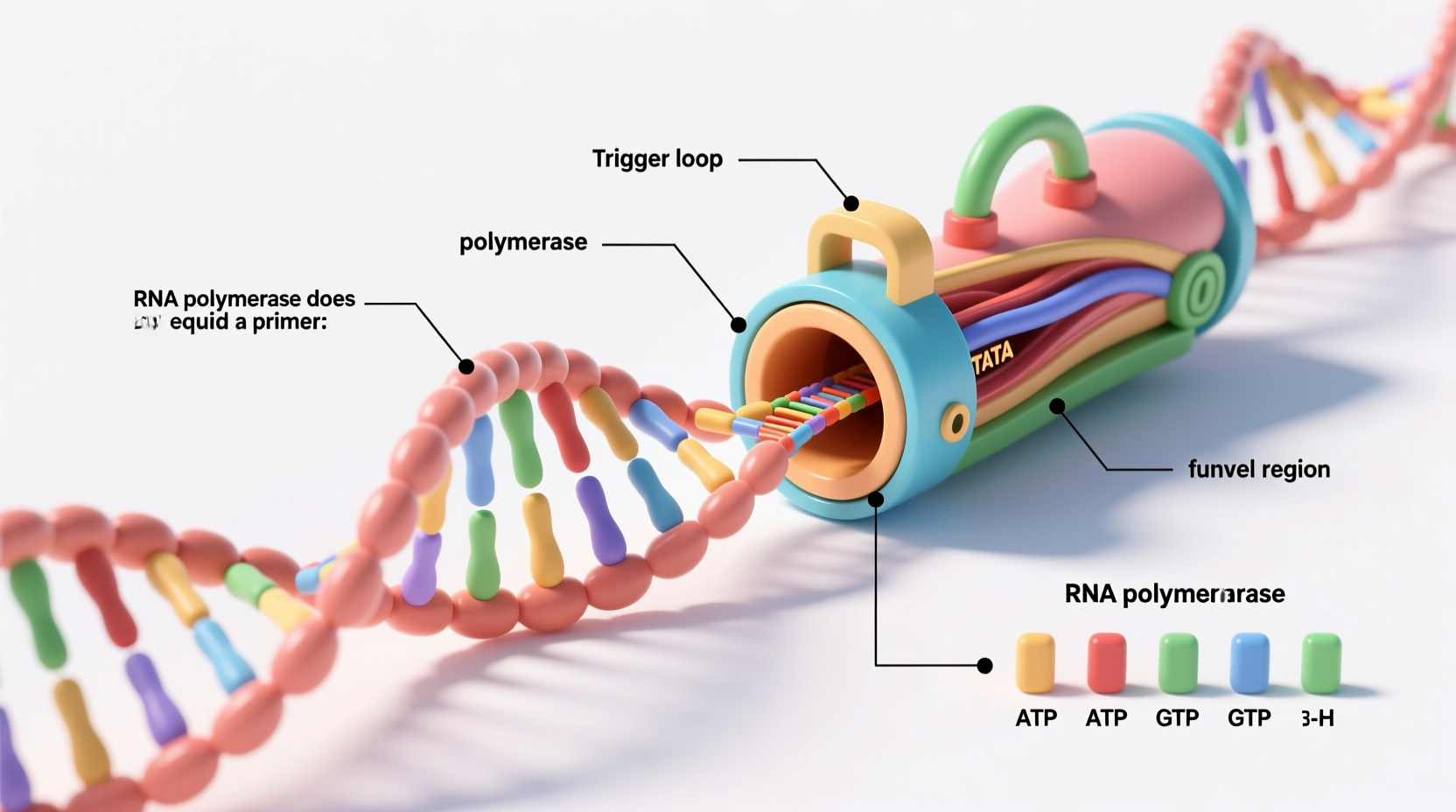
Transcription is the process by which genetic information encoded in DNA is copied into messenger RNA (mRNA), which then serves as a template for protein synthesis. The key enzyme driving this process is RNA polymerase. Unlike its counterpart in DNA replication, RNA polymerase can initiate nucleotide polymerization without a primer because it has the unique ability to recognize specific DNA sequences called promoters.
When RNA polymerase binds to a promoter region upstream of a gene, it unwinds a small portion of the DNA double helix, creating what’s known as the transcription bubble. Within this bubble, the enzyme selects the correct starting nucleotide—usually a purine (adenine or guanine)—and catalyzes the formation of the first phosphodiester bond between two ribonucleotides. This initial step does not rely on an existing 3'-OH group, which is required by DNA polymerases but absent during de novo initiation.
“RNA polymerase’s ability to start transcription without a primer allows cells to rapidly respond to environmental changes by initiating gene expression on demand.” — Dr. Laura Mendez, Molecular Biologist, University of California, San Diego
This independence from primers enables immediate response mechanisms. For example, when a cell encounters stress, such as heat shock, it must quickly activate protective genes. If each transcription event required a separate priming step, this response would be significantly delayed.
DNA Polymerase vs. RNA Polymerase: A Functional Comparison
The contrast between these two enzymes highlights their distinct biological roles. DNA polymerase functions in genome duplication, where accuracy and continuity are paramount. To ensure precision, it only adds nucleotides to an existing 3’ hydroxyl end—typically provided by a short RNA primer synthesized by primase. This mechanism prevents random initiation and reduces errors.
In contrast, RNA polymerase operates under different constraints. Its primary role isn’t to preserve the entire genome but to transcribe individual genes as needed. Therefore, speed, specificity, and regulatory control take precedence over continuous elongation mechanics.
| Feature | DNA Polymerase | RNA Polymerase |
|---|---|---|
| Primer Required? | Yes | No |
| Initiation Type | Primer-dependent | De novo |
| Product | Double-stranded DNA copy | Single-stranded RNA transcript |
| Proofreading Ability | High (3'→5' exonuclease) | Limited |
| Biological Role | Genome replication | Gene expression |
This functional divergence reflects evolutionary specialization: DNA polymerase ensures faithful inheritance, while RNA polymerase enables dynamic regulation of cellular activity.
The Role of Promoters and Transcription Factors
RNA polymerase achieves accurate initiation through interactions with promoter sequences and auxiliary proteins known as transcription factors. In bacteria, the sigma factor associates with RNA polymerase to form the holoenzyme, directing it to specific promoter motifs like the -10 (Pribnow box) and -35 elements. Once bound, the complex undergoes conformational changes that facilitate DNA melting and start site selection.
In eukaryotes, the process is more complex. Multiple general transcription factors (such as TFIID, TFIIB, and TFIIH) assemble at the promoter along with RNA polymerase II to form the pre-initiation complex. These proteins help unwind DNA, position the polymerase correctly, and regulate the timing of transcription onset—all without introducing a primer.
This elaborate system ensures that transcription begins precisely at the right location and time, compensating for the lack of a primer-based checkpoint by using sequence-specific recognition instead.
Step-by-Step: The Initiation Phase of Transcription
Understanding the mechanics of primer-independent initiation becomes clearer when broken down into stages:
- Promoter Recognition: RNA polymerase (with sigma factor in prokaryotes or transcription factors in eukaryotes) identifies and binds to the promoter region on DNA.
- DNA Melting: The enzyme locally unwinds about 12–14 base pairs of DNA, forming the transcription bubble.
- Start Site Selection: The polymerase aligns the first ribonucleoside triphosphate (usually ATP or GTP) complementary to the template strand.
- First Phosphodiester Bond Formation: The enzyme catalyzes the linkage between the first and second nucleotides, initiating RNA chain growth.
- Promoter Clearance: After synthesizing a short RNA segment (~10 nucleotides), RNA polymerase releases initiation factors and transitions into the elongation phase.
Each of these steps occurs without external priming, relying solely on the enzyme’s catalytic capacity and structural compatibility with the DNA template.
Mini Case Study: Heat Shock Response in E. coli
A real-world example illustrating the advantage of primer-independent transcription is the heat shock response in *Escherichia coli*. When exposed to sudden temperature increases, bacterial cells must rapidly produce chaperone proteins like DnaK and GroEL to prevent protein denaturation.
This response hinges on the alternative sigma factor σ32, which redirects RNA polymerase to heat shock gene promoters. Because no primers are needed, transcription of these protective genes begins within seconds of temperature elevation. If each transcript required a separate primase action and primer annealing step, the delay could prove fatal under extreme conditions.
This case underscores how the autonomy of RNA polymerase supports survival-critical responses across life forms.
Frequently Asked Questions
Why can’t DNA polymerase work without a primer?
DNA polymerase lacks the ability to initiate synthesis de novo because its active site requires a free 3'-hydroxyl group to add the next nucleotide. This structural constraint ensures high fidelity during replication by preventing random nucleotide additions. Primase provides a short RNA primer with this necessary 3'-OH end, allowing DNA polymerase to extend the strand accurately.
Does RNA polymerase ever use primers under special circumstances?
No, RNA polymerase does not use primers in natural cellular processes. Even in viral systems or laboratory settings like in vitro transcription, RNA polymerases (e.g., T7 RNA polymerase) initiate RNA synthesis without primers. Some specialized enzymes, such as telomerase (which contains an RNA component), perform primer extension, but this is mechanistically distinct from standard transcription.
Is the lack of proofreading in RNA polymerase related to not using a primer?
Not directly. The absence of a primer and limited proofreading are separate features. RNA polymerase trades some accuracy for speed and regulatory responsiveness. Since RNA transcripts are transient and multiple copies are made per gene, occasional errors are less consequential than in DNA replication, where mistakes become permanent mutations.
Key Takeaways and Best Practices for Understanding Transcription
- RNA polymerase initiates transcription de novo, eliminating the need for a primer.
- Promoter recognition and transcription factors ensure precise start site selection.
- Compared to DNA polymerase, RNA polymerase prioritizes regulatory control over absolute fidelity.
- The ability to begin without a primer enables rapid gene activation in response to stimuli.
- This mechanism is conserved across bacteria, archaea, and eukaryotes, highlighting its evolutionary importance.
Conclusion
The fact that RNA polymerase doesn’t need a primer is not a biochemical oversight but a sophisticated adaptation. It allows cells to launch gene expression swiftly, adapt to changing environments, and regulate thousands of genes independently and efficiently. By understanding this mechanism, we gain deeper appreciation for how life balances precision with flexibility at the molecular level. Whether you're exploring gene regulation, designing synthetic biology systems, or simply learning core biology concepts, recognizing the significance of de novo initiation empowers a more nuanced view of cellular function.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?