Air is essential to life on Earth, yet many people assume it’s a single substance. In reality, air is not a pure compound but a complex blend of gases, each playing a distinct role in the atmosphere. Understanding why air is classified as a mixture—rather than a compound—is fundamental to grasping basic chemistry and environmental science. This article explores the scientific reasoning behind this classification, supported by physical properties, compositional behavior, and experimental evidence.
Composition of Air: A Blend of Gases
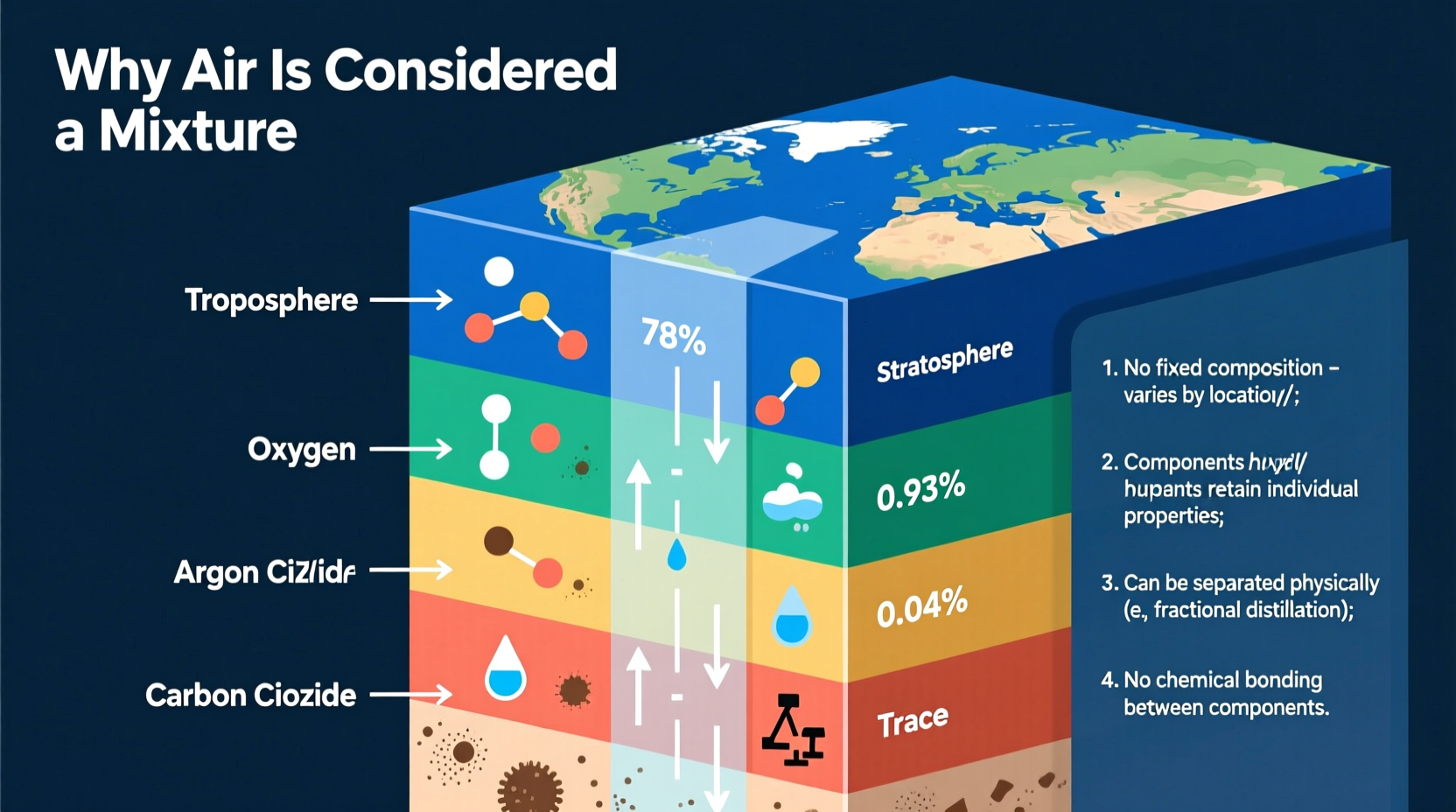
Air is primarily composed of nitrogen (about 78%), oxygen (around 21%), and trace amounts of argon, carbon dioxide, neon, helium, methane, and water vapor. These components coexist without undergoing chemical bonding, maintaining their individual properties. Unlike compounds such as water (H₂O), where hydrogen and oxygen chemically combine in fixed ratios, the gases in air mix physically and can vary slightly in proportion depending on location, altitude, and environmental conditions.
This variability itself is a strong indicator that air is a mixture. For example, near industrial zones, carbon dioxide levels may rise, while in forests, oxygen concentration can be marginally higher due to photosynthesis. Humidity also affects the amount of water vapor present, further demonstrating the non-fixed nature of air's composition.
Physical vs. Chemical Combination
One of the most critical distinctions between a mixture and a compound lies in how the components interact. In a compound, elements chemically bond to form a new substance with unique properties. For instance, sodium (a reactive metal) and chlorine (a toxic gas) combine to create table salt (NaCl), which is safe to consume.
In contrast, the gases in air do not react under normal atmospheric conditions. Nitrogen doesn’t chemically bind with oxygen; they simply occupy the same space independently. This lack of chemical bonding means no new substance is formed, and each gas retains its original chemical identity. You can separate oxygen from nitrogen through physical processes like fractional distillation of liquid air—something impossible if they were chemically bonded.
“Air behaves like a solution of gases. Its components can be separated by physical means, which is a hallmark of mixtures.” — Dr. Alan Reeves, Atmospheric Chemist
Key Properties Confirming Air as a Mixture
Several observable characteristics confirm that air is a mixture rather than a compound:
- No fixed ratio: While average percentages are well-documented, the actual composition varies globally and locally.
- Retention of individual properties: Oxygen supports combustion, nitrogen does not, and CO₂ turns limewater milky—all behaviors preserved in air.
- Separation by physical methods: Techniques like liquefaction and fractional distillation isolate gases without chemical reactions.
- No energy change upon mixing: When gases combine to form air, there’s no release or absorption of heat, unlike in chemical reactions.
- Reversibility: Components can be added or removed without altering the fundamental nature of the remaining gases.
Comparison: Mixture vs. Compound
| Property | Mixture (e.g., Air) | Compound (e.g., Water) |
|---|---|---|
| Composition | Variable proportions | Fixed ratio (H:O = 2:1) |
| Bonding | No chemical bonds | Covalent bonds present |
| Separation Method | Physical (distillation, diffusion) | Chemical (electrolysis) |
| Energy Change During Formation | Negligible | Significant (exothermic) |
| Properties of Components | Retained | Lost (new properties emerge) |
Real-World Example: Industrial Gas Separation
A practical demonstration of air’s status as a mixture occurs in industrial gas production. At cryogenic air separation plants, ambient air is filtered, compressed, cooled to -200°C, and then gradually warmed in a distillation column. As temperature rises, different gases boil off at their respective boiling points: nitrogen at -196°C, argon at -186°C, and oxygen at -183°C. This process cleanly separates high-purity oxygen and nitrogen for medical, welding, and aerospace applications.
If air were a compound, such separation would require breaking chemical bonds using large energy inputs, and the resulting substances would not be the original gases. Instead, we recover nitrogen and oxygen in their elemental forms—clear proof of physical mixing.
Common Misconceptions About Air
Some believe that because air appears uniform, it must be a compound. However, homogeneity does not imply chemical combination. Solutions like saltwater or alloys like brass are homogeneous mixtures too. Air is a homogeneous gaseous mixture—uniform at the macro level but composed of independent molecules.
Another misconception is that oxygen \"belongs\" to the air as a defining component. While vital for respiration, removing oxygen doesn’t destroy air—it simply changes its composition, much like removing sugar from tea doesn’t eliminate the drink.
Step-by-Step: How Scientists Prove Air Is a Mixture
- Collect a sample of dry air using a vacuum-sealed container to avoid contamination.
- Analyze gas composition via gas chromatography or spectroscopy to identify individual components.
- Cool the air gradually until it liquefies, observing multiple condensation points.
- Perform fractional distillation and collect fractions at different temperatures.
- Test each fraction chemically—for example, one will reignite a glowing splint (oxygen), another will extinguish flames (nitrogen).
- Conclude that since gases can be separated physically and retain their identities, air is a mixture.
Frequently Asked Questions
Can air become a compound under certain conditions?
No. While nitrogen and oxygen can react under extreme conditions—like lightning strikes—to form nitrogen oxides (NO, NO₂), these are minor, temporary products. The bulk of air remains unreacted. These reactions do not transform air into a compound; they merely introduce trace pollutants.
Why doesn’t the variable composition of air affect breathing?
Human lungs are adapted to function within typical atmospheric ranges. Small fluctuations in oxygen (20–21%) or CO₂ (<0.1%) have negligible effects. Only drastic changes—such as at high altitudes or in confined spaces—require physiological adjustments or supplemental oxygen.
Is polluted air still considered a mixture?
Yes. Even with added pollutants like sulfur dioxide or particulate matter, air remains a mixture. Pollutants disperse physically and don’t alter the fundamental classification. In fact, pollution highlights air’s ability to incorporate various substances without chemical integration.
Conclusion: Embracing the Complexity of Air
Air’s classification as a mixture is more than a textbook definition—it reflects a deeper understanding of how substances interact in nature. Its variable composition, physical blending, and separability all point to a dynamic system that supports life without rigid constraints. Recognizing air as a mixture allows scientists, educators, and environmentalists to better analyze atmospheric changes, develop clean technologies, and teach foundational chemistry concepts with real-world relevance.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?