In scientific inquiry, the pursuit of truth hinges on precision, objectivity, and reproducibility. One of the most fundamental tools researchers use to meet these standards is the control group. Whether in medicine, psychology, agriculture, or public policy, control groups serve as a benchmark against which experimental outcomes are measured. Without them, it becomes nearly impossible to determine whether observed effects stem from the treatment being tested—or from unrelated variables like chance, environment, or human bias.
The importance of control groups lies not just in their function but in their ability to isolate cause and effect. They allow researchers to answer a critical question: Did the intervention actually cause the change, or would the same result have occurred anyway?
The Purpose of a Control Group
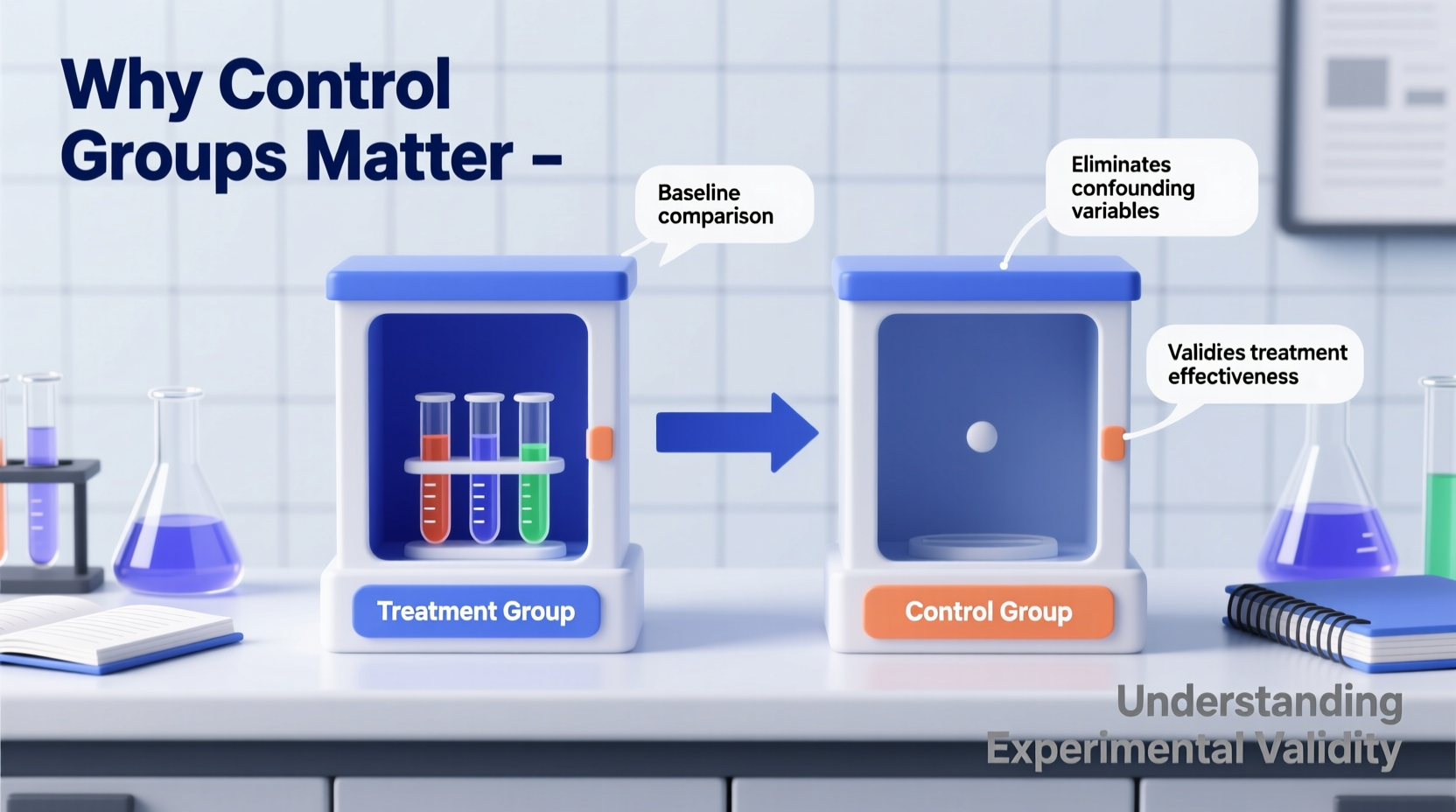
A control group consists of participants or subjects who do not receive the experimental treatment. Instead, they serve as a baseline for comparison. By holding all conditions constant except for the variable under investigation, scientists can attribute differences in outcomes directly to that variable.
For example, in a clinical trial testing a new drug for high blood pressure, one group receives the drug (the experimental group), while another receives a placebo (the control group). Both groups are monitored under identical conditions. If the drug group shows significantly greater improvement, researchers can reasonably conclude the drug is effective—provided other confounding factors were controlled.
This method eliminates assumptions and replaces them with evidence. It’s what separates rigorous science from anecdotal observation.
How Control Groups Prevent Bias and Error
One of the greatest challenges in research is minimizing bias—both conscious and unconscious. The placebo effect, for instance, demonstrates how expectation alone can influence outcomes. Patients given sugar pills may report symptom relief simply because they believe they’re receiving treatment.
Control groups help counteract this by allowing researchers to distinguish between psychological effects and physiological ones. When both groups are treated identically—except for the active ingredient—the difference in outcomes reflects the true impact of the intervention.
Blinding further strengthens this process. In double-blind studies, neither participants nor researchers know who is in the control or experimental group. This prevents expectations from influencing behavior or data interpretation.
Without a control group, researchers risk drawing false conclusions. A patient might improve after taking a new supplement, but was it the supplement—or rest, diet, or natural recovery? Only a properly designed study with a control group can answer that.
Types of Control Groups and Their Applications
Not all control groups are the same. Depending on the research question, different types are used to ensure accuracy and relevance.
| Type of Control Group | Description | Common Use Cases |
|---|---|---|
| Placebo Control | Participants receive an inert substance (e.g., sugar pill) | Medical trials where psychological effects are strong |
| Active Control | Participants receive a standard treatment already known to be effective | Testing a new drug against an existing one |
| No-Treatment Control | Participants receive no intervention at all | Behavioral or educational studies |
| Waitlist Control | Participants are promised the treatment later | Therapy or program evaluations with limited access |
Each type serves a strategic purpose. Placebo controls are powerful in isolating biological effects, while active controls help determine whether a new treatment is superior to current standards. Waitlist designs are ethically advantageous when withholding treatment entirely would be inappropriate.
“Control groups are not just a feature of good science—they are its foundation. Without them, we’re left guessing.” — Dr. Lena Patel, Biostatistician and Research Methodologist
Real-World Example: The Salk Polio Vaccine Trial
One of the most impactful uses of a control group occurred in the 1954 field trial of Jonas Salk’s polio vaccine. Over 1.8 million children participated, making it one of the largest medical experiments in history. Children were divided into two groups: one received the vaccine, the other a placebo.
The results were definitive. In communities where children received the real vaccine, polio rates dropped dramatically compared to the control group. This clear contrast provided irrefutable evidence of the vaccine’s effectiveness and led to widespread adoption.
Had there been no control group, public health officials could not have confidently attributed the decline in polio cases to the vaccine. Other factors—such as improved sanitation or seasonal variation—might have been blamed. The control group removed doubt and accelerated a medical breakthrough.
Step-by-Step: Designing an Effective Control Group
Creating a valid control group requires careful planning. Follow these steps to ensure reliability:
- Define the research question clearly. Know exactly what variable you’re testing.
- Randomly assign participants. Use randomization to distribute subjects across control and experimental groups, reducing selection bias.
- Ensure similarity in conditions. Both groups should experience the same environment, timing, and procedures—except for the treatment.
- Use blinding when possible. Prevent participants and researchers from knowing group assignments.
- Monitor adherence and dropouts. Track participation rates and reasons for withdrawal to assess data integrity.
- Analyze results comparatively. Use statistical methods to determine if differences between groups are significant.
Common Pitfalls and How to Avoid Them
Even well-intentioned studies can fail due to poor control group design. Common mistakes include:
- Non-random assignment: Leads to unequal distribution of characteristics (e.g., age, health status).
- Different treatment conditions: If the control group is handled differently, results become unreliable.
- Small sample size: Increases the risk of random variation skewing results.
- Contamination between groups: If members of the control group access the treatment, the comparison breaks down.
To avoid these issues, researchers must prioritize methodological rigor over convenience. Institutional review boards (IRBs) often require detailed protocols to prevent such errors before studies begin.
FAQ
Can a study work without a control group?
Sometimes, but with major limitations. Case studies or pre-post comparisons can suggest trends, but they cannot prove causation. Without a control group, it’s impossible to rule out alternative explanations for observed changes.
Is it ethical to withhold treatment from a control group?
Ethics depend on context. In life-threatening conditions, using a placebo may be unethical if an effective treatment exists. In such cases, an active control group—receiving the standard treatment—is preferred. Research ethics committees carefully evaluate these decisions to protect participants.
What if the control group improves without treatment?
This is common and informative. Improvement in the control group highlights the role of factors like natural recovery, placebo effects, or environmental influences. It underscores why comparison is essential—researchers measure the *additional* benefit of the treatment beyond these background effects.
Conclusion: The Backbone of Reliable Science
Control groups are more than a procedural detail—they are the cornerstone of credible experimentation. They transform speculation into evidence, opinion into data, and guesswork into knowledge. From pharmaceuticals to education reform, the presence of a well-designed control group determines whether a finding stands up to scrutiny or fades into irrelevance.
As consumers of information, we benefit from understanding this principle. When reading about a new study, ask: Was there a control group? How were participants assigned? What biases might remain? These questions empower us to discern real breakthroughs from misleading claims.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?