Ionic compounds like table salt (NaCl) appear stable as solids—crystalline, rigid, and held together by strong electrostatic forces. Yet drop a pinch into water, and it vanishes almost instantly. This dramatic transformation hinges on one key phenomenon: the weakening of ionic bonds in aqueous environments. While ionic bonds are among the strongest in solid form, they become surprisingly fragile when surrounded by water molecules. Understanding this shift is essential for grasping everything from biological ion transport to industrial desalination.
The Nature of Ionic Bonds
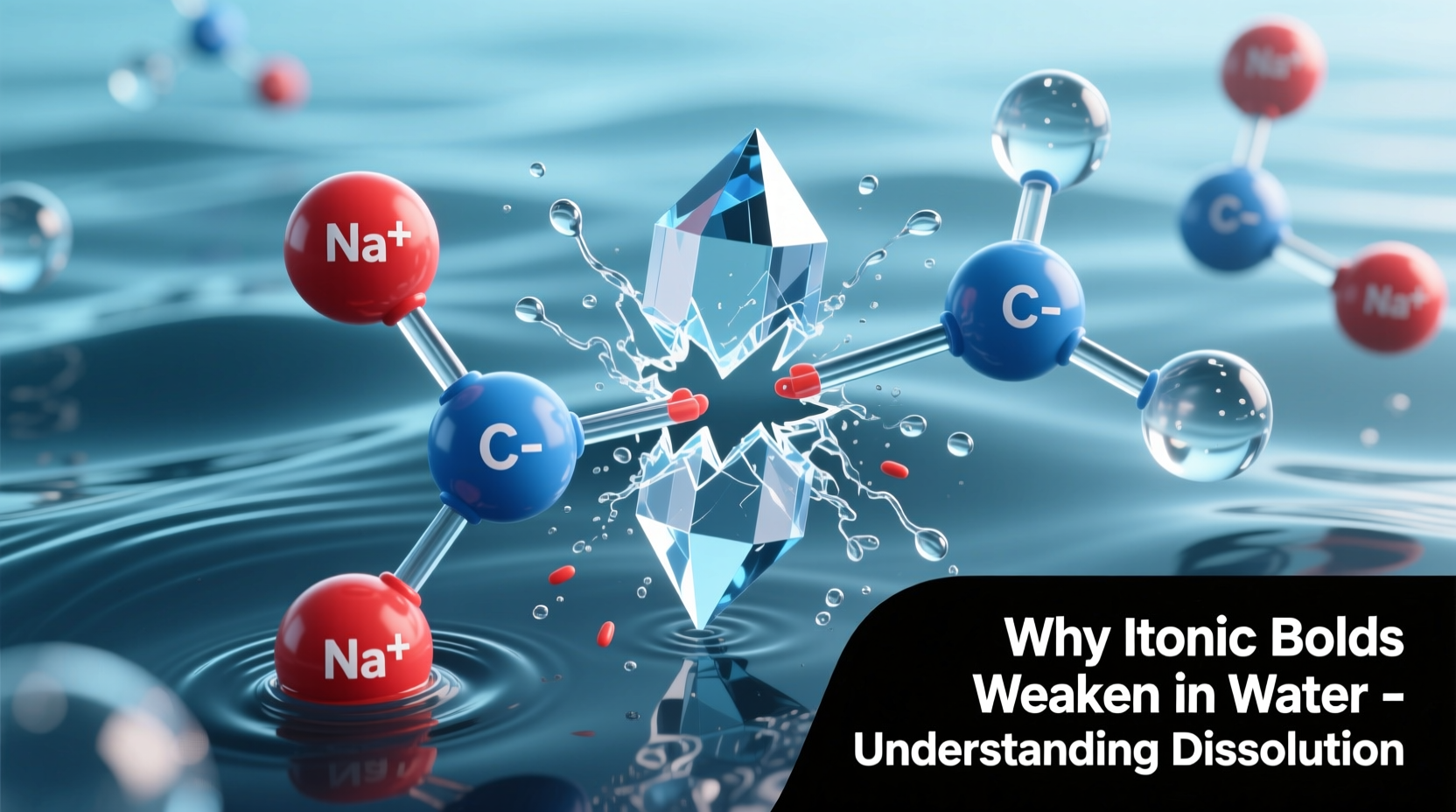
Ionic bonds form when electrons transfer completely from one atom to another, typically between metals and nonmetals. Sodium donates an electron to chlorine, creating Na⁺ and Cl⁻ ions that attract each other through powerful electrostatic forces. In a crystal lattice, these alternating charges create a highly ordered, stable structure with high melting points and brittleness.
Despite their strength in dry, solid conditions, ionic compounds often dissolve readily in water. This seems contradictory—how can such strong bonds break so easily? The answer lies not in the bond itself weakening, but in the environment’s ability to counteract the attraction between ions.
Water’s Unique Molecular Structure
Water (H₂O) is a polar molecule. Oxygen is more electronegative than hydrogen, pulling electron density toward itself. This creates a partial negative charge (δ⁻) on the oxygen and partial positive charges (δ⁺) on the hydrogens. The bent shape of the molecule amplifies this polarity, giving water a significant dipole moment.
This polarity allows water to interact strongly with charged particles. When an ionic compound enters water, the positive ends (hydrogens) of water molecules surround anions, while the negative ends (oxygens) orient toward cations. These interactions are known as ion-dipole forces—and they’re powerful enough to compete with ionic bonds.
How Dissolution Breaks the Lattice
Dissolution isn’t a simple breaking of ionic bonds—it’s a competition between two forces:
- The electrostatic attraction holding ions in the crystal lattice.
- The cumulative ion-dipole attractions between ions and surrounding water molecules.
When NaCl enters water, H₂O molecules begin clustering around exposed Na⁺ and Cl⁻ ions at the crystal surface. Each Na⁺ becomes surrounded by water molecules oriented with their oxygen atoms inward; each Cl⁻ attracts hydrogens. This process is called hydration, and the resulting clusters are hydration shells.
If the total energy released by forming hydration shells exceeds the lattice energy (the energy required to separate ions in the solid), the net process is energetically favorable—and dissolution proceeds spontaneously.
Lattice Energy vs. Hydration Energy
Lattice energy depends on ion charge and size: higher charges and smaller ions increase lattice strength. For example, MgO has far greater lattice energy than NaCl due to 2+ and 2− charges. Hydration energy follows similar trends—smaller, highly charged ions attract water more strongly.
The balance between these energies determines solubility:
| Compound | Lattice Energy (kJ/mol) | Hydration Energy (kJ/mol) | Net Energy Change | Soluble? |
|---|---|---|---|---|
| NaCl | +787 | −784 | −3 (slightly exothermic) | Yes |
| MgO | +3795 | −3920 | −125 | No (insoluble) |
| KBr | +682 | −679 | −3 | Yes |
Note: Even though MgO has a large negative net energy, its extremely high lattice energy makes it kinetically insoluble under normal conditions. Solubility isn't only about thermodynamics—it also involves activation barriers and entropy changes.
“Water doesn’t destroy ionic bonds—it outcompetes them. The collective strength of hydration often exceeds the pull of the lattice.” — Dr. Alan Reyes, Physical Chemist, MIT
Step-by-Step: The Dissolution Process
Understanding dissolution as a dynamic process helps clarify why ionic bonds seem “weak” in water:
- Contact: The ionic crystal comes into contact with water molecules.
- Surface Interaction: Water dipoles align with surface ions, exerting attractive forces.
- Ion Separation: If hydration energy > lattice energy, ions detach from the lattice.
- Hydration Shell Formation: Released ions become encased in layers of oriented water molecules.
- Diffusion: Hydrated ions disperse throughout the solution, preventing recombination.
This sequence explains why stirring accelerates dissolution: it removes hydrated ions from the surface, maintaining a concentration gradient that favors further ion release.
Why Don’t All Ionic Compounds Dissolve?
Not all ionic substances vanish in water. Silver chloride (AgCl), for instance, remains largely undissolved. Its lattice energy is high due to covalent character and small ion sizes, while hydration cannot compensate sufficiently. Additionally, entropy plays a role—some systems favor order over dispersion.
Real-World Implications: From Biology to Industry
The principle of weakened ionic bonds in water underpins critical processes across disciplines.
Mini Case Study: Electrolyte Balance in Blood
In human physiology, dissolved ions—Na⁺, K⁺, Ca²⁺, Cl⁻—are essential for nerve signaling, muscle contraction, and osmotic balance. These ions exist freely in plasma not because their original bonds were weak, but because water effectively shields them from recombination. When patients receive IV saline, NaCl dissociates instantly into mobile ions thanks to hydration.
Disruptions in this system—such as dehydration—reduce water availability, increasing ion concentration and altering electrical gradients. This demonstrates how solvent quantity directly affects ion behavior, even if bond strength remains unchanged.
Industrial Application: Desalination
Reverse osmosis plants remove salt from seawater by forcing it through semi-permeable membranes. The process works because hydrated ions are too large or repelled by the membrane, while water molecules pass through. This relies entirely on the fact that ions remain separated due to hydration shells—a direct consequence of water overcoming ionic lattice stability.
Common Misconceptions Clarified
- Misconception: “Ionic bonds become weaker in water.”
Reality: The bond strength doesn’t change—the surrounding water simply provides a more favorable energetic alternative via hydration. - Misconception: “All ionic compounds dissolve in water.”
Reality: Solubility depends on the balance of lattice and hydration energies. Many ionic compounds (e.g., BaSO₄) are insoluble. - Misconception: “Dissolved ions are free-floating.”
Reality: Ions are always surrounded by hydration shells—typically 4–6 tightly bound water molecules.
Frequently Asked Questions
Does water break ionic bonds?
Not exactly. Water molecules use ion-dipole forces to pull ions away from the crystal lattice. The energy from hydration compensates for the energy needed to separate ions, making dissolution favorable. The ionic bond isn’t “broken” like a snapped stick—it’s outcompeted.
Are ionic bonds weak in general?
No. In solid form, ionic bonds are very strong—evidenced by high melting points (NaCl melts at 801°C). Their apparent weakness in water is due to the exceptional solvating power of H₂O, not inherent bond instability.
Can ionic compounds dissolve in nonpolar solvents?
Rarely. Nonpolar solvents lack dipole moments and cannot stabilize ions. Without hydration-like interactions, lattice energy dominates, preventing dissolution. This is why salt doesn’t dissolve in oil or hexane.
Action Checklist: Understanding Ionic Dissolution
To master this concept, follow these steps:
- Identify the ions present in a given ionic compound.
- Sketch how water molecules would orient around each ion.
- Compare expected lattice and hydration energies based on ion size and charge.
- Predict solubility using general rules (e.g., most nitrates are soluble).
- Explain dissolution in terms of energy competition, not bond destruction.
Conclusion
The apparent weakness of ionic bonds in water is a testament to water’s extraordinary properties—not a flaw in ionic bonding. Through hydration, water stabilizes individual ions more effectively than the crystal lattice can, leading to dissolution. This principle governs chemical behavior in oceans, cells, batteries, and industrial reactors. Recognizing that it’s not the bond that weakens, but the environment that adapts, transforms confusion into clarity.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?