DNA replication is one of the most fundamental processes in biology, ensuring that genetic information is faithfully passed from one generation of cells to the next. A key feature of this process is the directional addition of nucleotides—specifically, they are added only to the 3' end of a growing DNA strand. This might seem like a minor biochemical detail, but it has profound implications for how life stores and transmits information. Understanding why nucleotides are added exclusively to the 3' end reveals deep insights into enzyme function, molecular stability, and evolutionary design.
The Chemistry Behind Nucleotide Addition
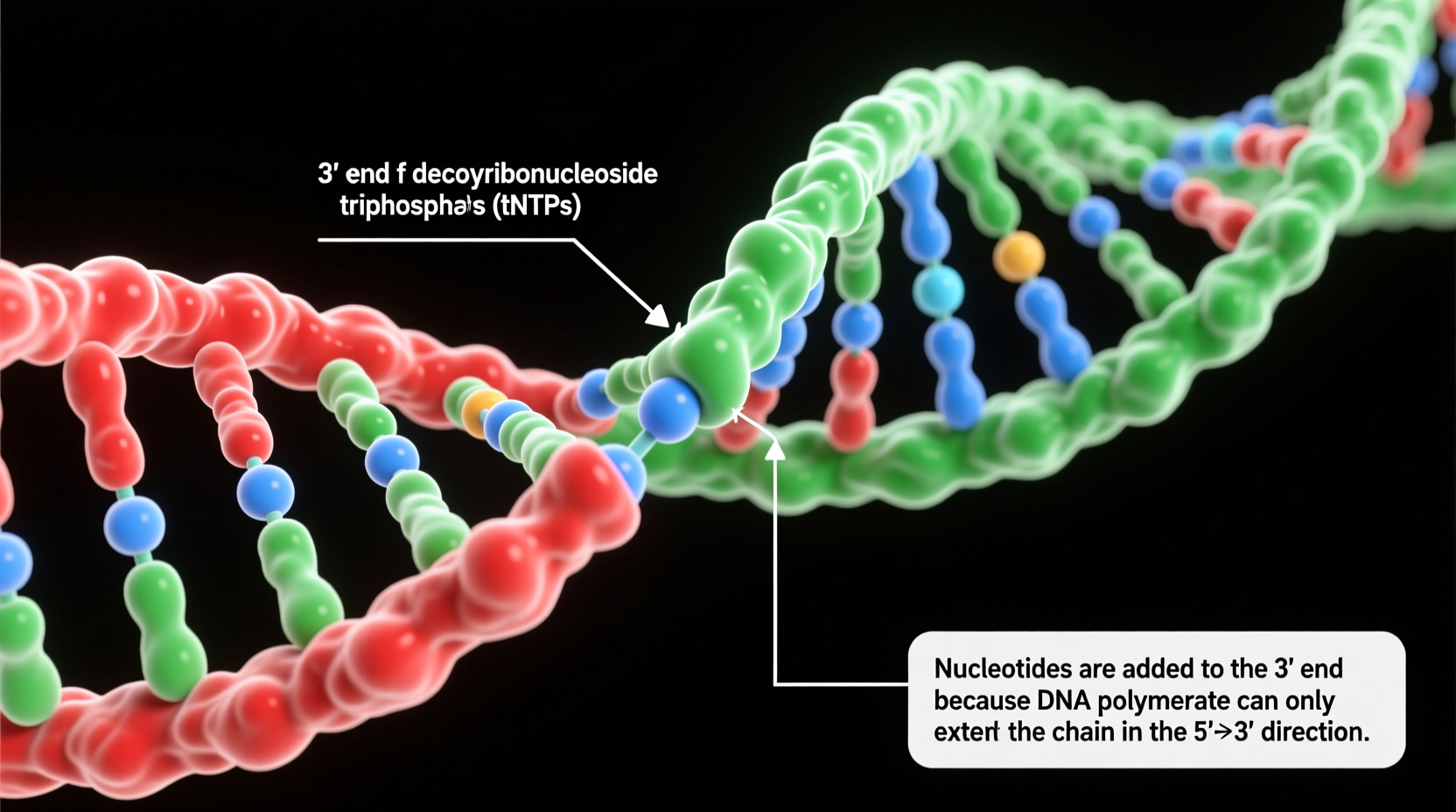
DNA polymerase, the primary enzyme responsible for synthesizing new DNA strands, can only add nucleotides in one direction: from the 5' phosphate group of an incoming nucleotide to the 3' hydroxyl (–OH) group of the last nucleotide in the chain. This reaction forms a phosphodiester bond, which links the sugar of one nucleotide to the phosphate of the next, creating the sugar-phosphate backbone of DNA.
This chemical constraint arises because DNA polymerase requires a free 3'–OH group to initiate a nucleophilic attack on the alpha phosphate of the incoming deoxyribonucleoside triphosphate (dNTP). The energy released by cleaving off two phosphate groups (pyrophosphate) drives the formation of the new bond. Without a free 3'–OH, no elongation can occur.
Why Not the 5' End? Structural and Functional Constraints
One might wonder why evolution didn’t favor growth at the 5' end. The answer lies in both chemistry and error correction. The 3' end provides a reactive hydroxyl group essential for catalysis, whereas the 5' end typically carries a phosphate group that is not nucleophilic enough to initiate bond formation under physiological conditions.
Moreover, having a consistent direction of synthesis allows DNA polymerase to incorporate proofreading functions. Most DNA polymerases have a 3' → 5' exonuclease activity that scans the newly added nucleotide immediately after incorporation. If a mismatch is detected, the enzyme removes the incorrect base from the 3' end before continuing synthesis. This mechanism would be impossible if the chain grew from the 5' end, as there would be no accessible 3' terminus for excision.
“Directional synthesis isn't arbitrary—it’s a masterpiece of molecular engineering that enables both speed and fidelity.” — Dr. Linda Chen, Molecular Biologist, Stanford University
The Antiparallel Nature of DNA and Its Impact on Replication
DNA strands are antiparallel—one runs 5' → 3', and its complement runs 3' → 5'. Because DNA polymerase only works in the 5' → 3' direction, this creates a challenge: while one new strand (the leading strand) can be synthesized continuously in the direction of the replication fork, the other (the lagging strand) must be made in short, discontinuous fragments known as Okazaki fragments.
These fragments are later joined by DNA ligase to form a continuous strand. This asymmetry is a direct consequence of the 3'-end addition rule and highlights how a single biochemical limitation shapes the entire architecture of replication machinery.
| Strand Type | Synthesis Direction | Continuity | Key Enzymes Involved |
|---|---|---|---|
| Leading Strand | 5' → 3' (continuous) | Continuous | DNA polymerase III, primase |
| Lagging Strand | 5' → 3' (in segments) | Discontinuous (Okazaki fragments) | DNA polymerase III, ligase, RNase H |
Step-by-Step: How Nucleotides Are Added During Replication
The process of adding nucleotides to the 3' end follows a precise sequence governed by multiple proteins and enzymes. Here's a simplified timeline of events at the replication fork:
- Helicase unwinds the double helix, separating the two template strands.
- Single-strand binding proteins (SSBs) stabilize the exposed single strands.
- Primase synthesizes a short RNA primer (typically 5–10 nucleotides), providing a free 3'–OH group.
- DNA polymerase III binds to the primer and begins adding DNA nucleotides to the 3' end.
- Elongation proceeds continuously on the leading strand but in bursts on the lagging strand.
- RNase H and DNA polymerase I replace RNA primers with DNA.
- DNA ligase seals nicks between Okazaki fragments.
This coordinated cascade ensures high-fidelity duplication of the genome, all made possible by the directional constraint of 3'–OH dependence.
Biological Advantages of 5' → 3' Synthesis
Beyond chemical feasibility, synthesizing DNA in the 5' → 3' direction offers several biological advantages:
- Error correction: As mentioned, the 3' → 5' exonuclease activity allows real-time proofreading, reducing mutation rates by up to 100-fold.
- Energy efficiency: The exergonic release of pyrophosphate during nucleotide incorporation helps drive the reaction forward without requiring additional ATP at each step.
- Coordination with transcription: In many organisms, genes are transcribed in the same direction as replication, minimizing collisions between machineries.
- Evolutionary conservation: All known cellular life forms use 5' → 3' synthesis, suggesting strong selective pressure for this mechanism.
Mini Case Study: Mutation Due to Impaired 3' Processing
In a 2018 study published in *Nature Genetics*, researchers investigated a rare hereditary cancer syndrome linked to a mutation in the proofreading domain of DNA polymerase epsilon. Patients carried a defective version of the enzyme that could still add nucleotides to the 3' end but failed to remove mispaired bases efficiently.
As a result, somatic cells accumulated mutations at an accelerated rate, particularly in tumor suppressor genes. This case illustrates how critical the 3' end isn’t just for chain extension—but also for maintaining genomic integrity. Even when nucleotide addition works, the absence of proper 3' monitoring leads to devastating consequences.
Frequently Asked Questions
Can DNA polymerase add nucleotides to the 5' end?
No. DNA polymerase cannot initiate or extend DNA chains at the 5' end due to the lack of a free 3'–OH group required for catalysis. All known DNA polymerases synthesize DNA exclusively in the 5' → 3' direction.
Why does the lagging strand exist?
The lagging strand exists because DNA polymerase only adds nucleotides to the 3' end, and the two original DNA strands run in opposite directions. Since the replication fork moves in one direction, only one new strand can be synthesized continuously. The other must be built backward in segments.
What happens if the 3' end is damaged?
A damaged 3' end—such as one missing the hydroxyl group—cannot support further elongation. Cells have repair mechanisms, including exonuclease trimming and template switching, to restore functional ends before replication resumes.
Checklist: Key Concepts in 3'-End Nucleotide Addition
- ✅ DNA polymerase requires a free 3'–OH group to add nucleotides.
- ✅ Synthesis always proceeds in the 5' → 3' direction.
- ✅ The antiparallel structure of DNA necessitates leading and lagging strands.
- ✅ Proofreading occurs via 3' → 5' exonuclease activity.
- ✅ RNA primers provide the initial 3'–OH for DNA polymerase to begin.
- ✅ Disruption of 3' processing can lead to mutations and disease.
Conclusion
The fact that nucleotides are added to the 3' end during DNA replication is far more than a textbook footnote—it is a cornerstone of molecular biology. This directional specificity enables accurate copying, efficient proofreading, and evolutionary stability across billions of cell divisions. From the chemistry of phosphodiester bonds to the orchestration of replication forks, every aspect hinges on the reactivity of the 3' hydroxyl group.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?