Gases are all around us—filling our lungs, powering engines, and enabling industrial processes. One of their most defining characteristics is the ability to be compressed. Unlike solids or liquids, gases can shrink dramatically under pressure, making them ideal for storage and transport in confined spaces. But what exactly makes this possible? The answer lies in the nature of gas molecules, the forces between them, and the vast empty space that separates them. Understanding gas compressibility isn’t just a textbook concept—it has practical implications in engineering, medicine, energy, and everyday life.
The Molecular Basis of Gas Compressibility
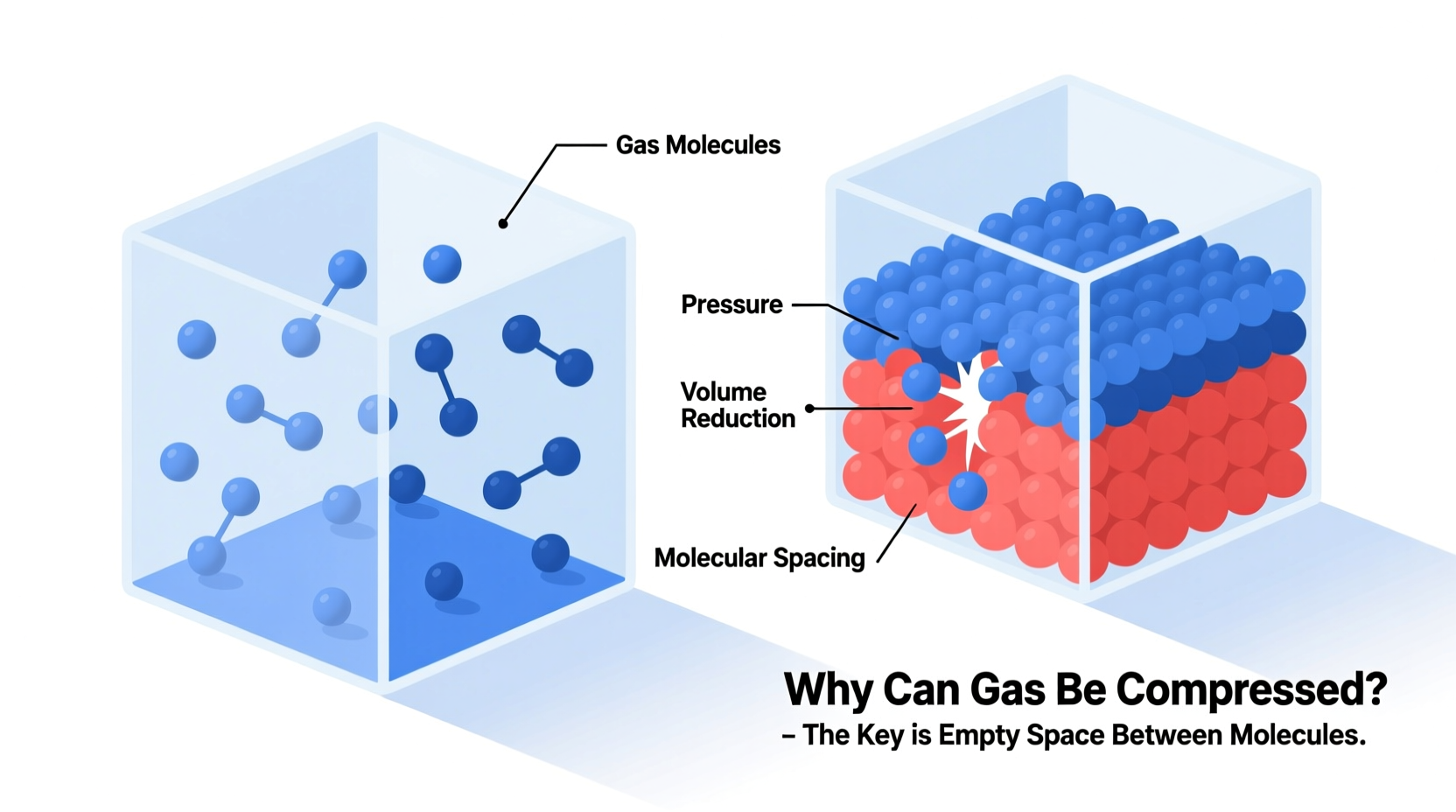
Gases consist of molecules that are in constant, rapid motion. These molecules move freely and independently, colliding with each other and the walls of their container. What sets gases apart from solids and liquids is the amount of empty space between individual molecules. In a typical gas at room temperature and atmospheric pressure, molecules occupy less than 0.1% of the total volume—the rest is empty space.
This large intermolecular distance means there’s significant room to push the molecules closer together when external pressure is applied. When you compress a gas, you’re not reducing the size of the molecules themselves; you’re simply decreasing the space between them. This contrasts sharply with liquids and solids, where molecules are already tightly packed, leaving little room for further compression.
“Gases are compressible because their particles are widely separated. That spacing allows us to reduce volume significantly under pressure.” — Dr. Alan Reyes, Physical Chemist
Factors Affecting Gas Compressibility
While all gases are compressible to some degree, the extent varies based on several physical conditions. Understanding these factors helps predict how a gas will behave under different environments.
- Pressure: Increasing pressure forces gas molecules closer together, reducing volume. At very high pressures, deviations from ideal behavior become noticeable due to intermolecular attractions.
- Temperature: Higher temperatures increase molecular kinetic energy, causing molecules to resist compression. Cooling a gas makes it easier to compress.
- Type of Gas: Lighter gases like hydrogen and helium behave more ideally and are highly compressible. Heavier gases with stronger intermolecular forces (like butane) deviate more from ideal gas behavior at high pressures.
- Volume of Container: The compressibility effect is most apparent when gas is forced into a smaller container, such as a propane tank or scuba cylinder.
Real-World Applications of Compressed Gases
The ability to compress gases enables countless technologies we rely on daily. Here are a few key examples:
- Medical Oxygen Tanks: Hospitals store oxygen in compressed form so it can be delivered efficiently to patients via masks or ventilators.
- Liquefied Petroleum Gas (LPG): Propane and butane are compressed and stored in liquid form for use in cooking, heating, and vehicles.
- Scuba Diving: Air or specialized gas mixtures are compressed into steel or aluminum cylinders, allowing divers to breathe underwater for extended periods.
- Pneumatic Tools: Nail guns, jackhammers, and air compressors operate using stored compressed air to generate mechanical force.
- Natural Gas Transportation: Methane is compressed (CNG) or liquefied (LNG) for efficient pipeline and tanker transport across long distances.
Mini Case Study: Compressed Natural Gas in Public Transit
In cities like Delhi and Tehran, public buses run on compressed natural gas (CNG) instead of diesel. CNG is stored in high-pressure tanks (typically 200–250 bar) on the vehicle roof. By compressing methane, operators achieve a usable fuel density without the need for cryogenic cooling. This switch has reduced urban air pollution significantly while maintaining engine performance. The success of CNG fleets demonstrates how compressibility enables cleaner energy transitions in transportation.
The Ideal Gas Law and Deviations
The relationship between pressure, volume, temperature, and the number of moles of gas is described by the Ideal Gas Law: PV = nRT. This equation assumes that gas molecules have no volume and experience no intermolecular forces—conditions that hold reasonably well at low pressures and high temperatures.
However, real gases deviate from ideality under extreme conditions. At high pressures, the actual volume of molecules becomes significant compared to the container size. Additionally, attractive forces between molecules cause the measured pressure to be lower than predicted. To account for these effects, scientists use equations like the van der Waals equation:
(P + an²/V²)(V - nb) = nRT
Here, 'a' corrects for intermolecular attraction, and 'b' accounts for the finite volume of gas molecules. These adjustments make predictions more accurate for real-world applications involving high-pressure systems.
Do’s and Don’ts of Handling Compressed Gases
| Do’s | Don’ts |
|---|---|
| Store cylinders upright and secured | Drop or roll gas cylinders |
| Use pressure regulators appropriate for the gas type | Expose tanks to heat sources or direct sunlight |
| Inspect valves and connections regularly | Mix incompatible gases in the same system |
| Cool gases before compression when possible | Overfill containers beyond rated pressure limits |
Step-by-Step: How Gas Compression Works in Practice
Consider the process of filling a scuba tank—a practical example of gas compression in action.
- Preparation: Inspect the tank for damage and ensure the valve is clean and functional.
- Initial State: The tank is open to atmosphere, filled with air at ~1 atm pressure.
- Connection: Attach the tank to a high-pressure compressor outlet using a secure fitting.
- Compression Phase: The compressor draws in ambient air, filters it, and progressively increases pressure through multiple stages (often up to 200–300 bar).
- Heat Management: Intercoolers between stages remove heat generated during compression to prevent overheating and improve efficiency.
- Filling Completion: Once the desired pressure is reached, the system shuts off automatically.
- Safety Check: The tank is tested for leaks and labeled with fill date and pressure rating.
This entire process relies on the fundamental principle that gas molecules can be forced into a much smaller volume when energy (in the form of mechanical work) is applied.
FAQ
Why can't liquids be compressed like gases?
Liquids have molecules that are already close together, with strong intermolecular forces. Applying pressure results in minimal volume change because there's little empty space left between molecules.
Is compressed gas dangerous?
Compressed gases can be hazardous if mishandled. High-pressure tanks may rupture if damaged, overheated, or improperly stored. Always follow safety guidelines and use appropriate protective equipment.
Can all gases be liquefied by compression alone?
No. A gas must be below its critical temperature to liquefy through compression. For example, carbon dioxide can be liquefied at room temperature under pressure, but nitrogen requires cooling first.
Expert Insight: Why Compressibility Matters Beyond Physics
“Gas compressibility isn’t just a lab curiosity—it’s foundational to energy storage, climate technology, and even space exploration. Think about inflating a Mars habitat or storing hydrogen fuel. Without understanding compressibility, modern engineering would stall.” — Dr. Lena Patel, Aerospace Materials Scientist
Conclusion
The reason gas can be compressed lies in its molecular structure: widely spaced particles with negligible intermolecular forces allow for dramatic volume reduction under pressure. From life-saving medical devices to sustainable transportation, the science of compressibility powers innovation across industries. As energy systems evolve toward cleaner alternatives like hydrogen and biogas, mastering gas compression will only grow in importance.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?