When you sip a sports drink after a workout or dissolve an electrolyte tablet in water, there’s no mistaking that distinct tang—salty, slightly metallic, unmistakably sharp. But why do electrolytes taste salty? It’s not just a coincidence; it’s rooted in human biology, chemistry, and evolutionary adaptation. Electrolytes like sodium, potassium, and chloride don’t just support hydration—they interact directly with your taste system in ways that make saltiness their signature flavor. Understanding this connection reveals more than just how we perceive taste; it sheds light on how our bodies maintain balance, respond to dehydration, and even crave certain minerals.
The Chemistry Behind Electrolytes and Taste
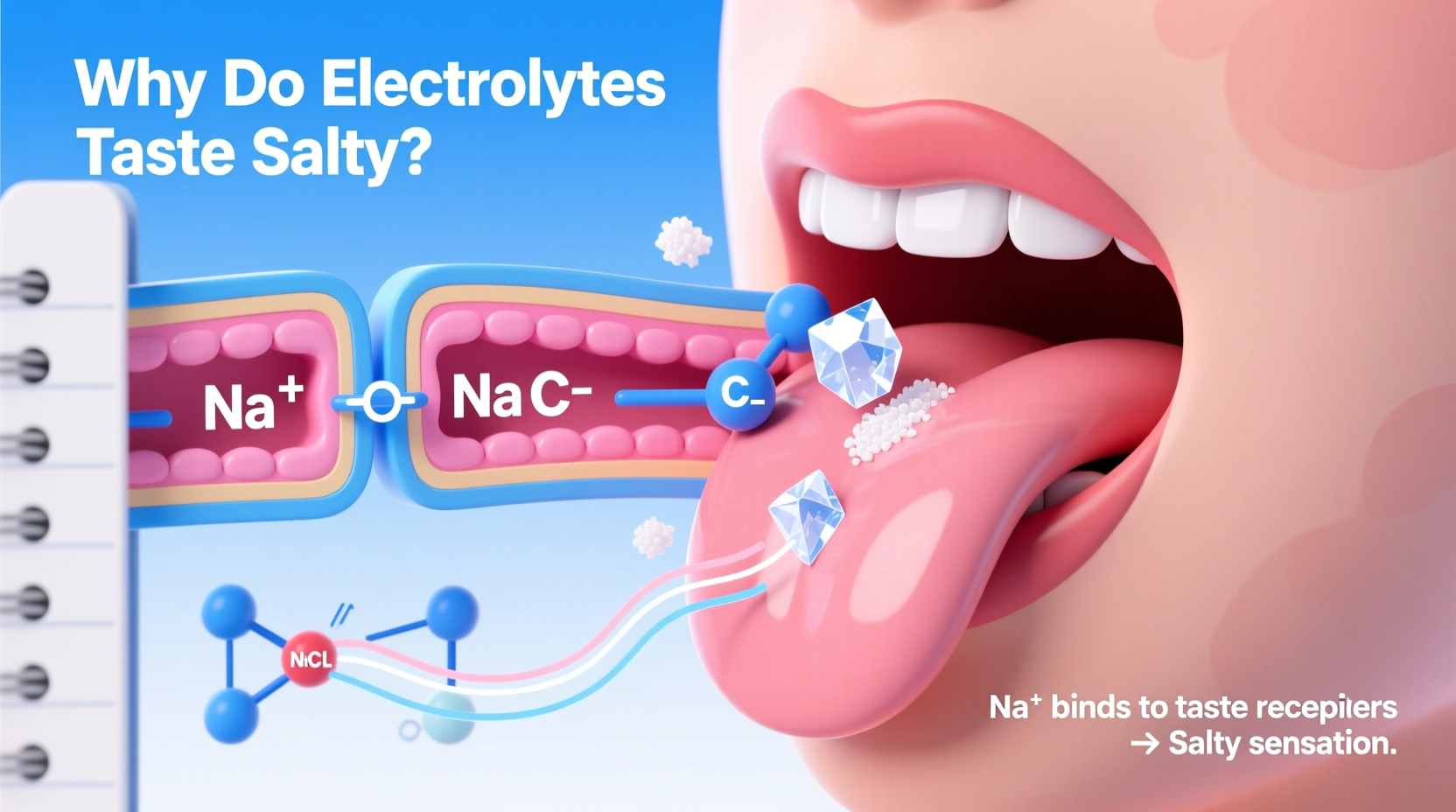
Electrolytes are minerals that carry an electric charge when dissolved in water. The most common ones in the human body include sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), magnesium (Mg²⁺), chloride (Cl⁻), bicarbonate (HCO₃⁻), and phosphate (PO₄³⁻). These ions play critical roles in nerve signaling, muscle contraction, fluid balance, and pH regulation.
When you consume an electrolyte-rich solution, these charged particles dissociate in liquid and become available to interact with your taste buds. Among them, sodium and chloride are the primary contributors to the salty taste. Sodium ions (Na⁺) in particular bind to specific receptors on the tongue, triggering a neural signal interpreted by the brain as “salt.” This isn't just a random sensory experience—it's a highly evolved mechanism designed to guide humans toward essential nutrients.
“Salt taste is one of the five basic tastes because it signals the presence of sodium, a mineral vital for survival. Our sensitivity to it is hardwired.” — Dr. Linda Bartoshuk, Professor of Dentistry and Taste Specialist, University of Florida
How Your Tongue Detects Saltiness
Taste perception begins in the taste buds, clusters of specialized cells located primarily on the tongue. Each taste bud contains receptor cells tuned to one of five basic tastes: sweet, sour, bitter, umami, and salty. The detection of salt relies heavily on epithelial sodium channels (ENaC), which are found in taste receptor cells on the front and sides of the tongue.
When sodium ions from a solution enter these channels, they generate an electrical signal that travels via nerves to the brainstem and then to the gustatory cortex. The brain interprets this signal as saltiness. Notably, ENaC channels are most sensitive to sodium, which explains why table salt (sodium chloride) tastes saltier than other electrolyte salts like potassium chloride—even though both contain chloride.
Potassium can also contribute to saltiness, but it often comes with a bitter or metallic aftertaste because it activates bitter receptors as well. This dual activation makes high-potassium solutions less palatable, which is why many low-sodium products struggle with consumer acceptance despite being healthier.
Why Evolution Favored a Craving for Salt
The human preference for salt is not accidental. Throughout evolution, dietary sodium was scarce. Early humans consumed plant-based diets low in sodium, while physical exertion and heat exposure caused significant sodium loss through sweat. A biological drive to seek out salt increased survival odds by ensuring adequate intake of this critical electrolyte.
This evolutionary pressure fine-tuned our taste system to detect even minute concentrations of sodium. In fact, humans can detect salt at levels as low as 0.2% concentration—making it one of the most sensitive taste modalities. That sensitivity once helped us locate natural salt sources like mineral-rich soil or animal blood. Today, however, that same instinct contributes to overconsumption in environments where salt is abundant and cheap.
Despite modern access to salt, the body still regulates sodium tightly through the kidneys and hormones like aldosterone. When sodium levels drop, the brain triggers cravings. This feedback loop reinforces why electrolyte drinks often taste satisfying during or after intense activity—they’re replenishing what the body urgently needs.
Electrolyte Composition and Flavor Profiles
Not all electrolyte sources taste the same, even if they serve similar physiological functions. The flavor depends on the type and ratio of ions present. Below is a comparison of common electrolyte compounds and their taste characteristics:
| Compound | Primary Ions | Taste Profile | Common Use |
|---|---|---|---|
| Sodium Chloride (NaCl) | Na⁺, Cl⁻ | Clean, sharp saltiness | Table salt, rehydration solutions |
| Potassium Chloride (KCl) | K⁺, Cl⁻ | Salty with bitter/metallic aftertaste | Salt substitute, medical supplements |
| Sodium Citrate | Na⁺, citrate³⁻ | Mild saltiness with slight tartness | Oral rehydration, buffered drinks |
| Magnesium Sulfate (Epsom salt) | Mg²⁺, SO₄²⁻ | Bitter, unpleasant | Laxative, not for regular consumption |
| Calcium Carbonate | Ca²⁺, CO₃²⁻ | Chalky, slightly salty | Dietary supplement, antacids |
The dominance of sodium in commercial electrolyte products isn’t arbitrary—it’s driven by palatability. Formulators aim to deliver effective hydration without triggering aversion. That’s why most sports drinks rely on sodium chloride or sodium citrate rather than alternatives that might cause bitterness or off-flavors.
Real-World Example: Athlete Hydration Strategy
Consider marathon runner Maria Chen, who began experiencing cramps during long-distance races despite drinking plenty of water. Her coach suggested she switch from plain water to an electrolyte beverage containing sodium, potassium, and magnesium. Initially, she disliked the salty taste, finding it medicinal compared to her usual flavored drinks.
After adjusting the concentration and adding lemon juice to mask the sharpness, Maria noticed a dramatic improvement—not only in performance but also in recovery. Over time, her palate adapted, and she began to associate the salty taste with relief and stamina. “Now,” she says, “if my drink doesn’t have that slight salt kick, I feel like I’m not getting enough support.”
Her experience illustrates how taste can serve as a functional cue. The saltiness wasn’t just a side effect—it was a signal that essential minerals were being replenished.
Do’s and Don’ts of Managing Electrolyte Taste
- Do dilute concentrated electrolyte powders if the taste is too strong.
- Do pair electrolyte drinks with citrus or berry flavors to balance saltiness.
- Do choose products with balanced sodium-potassium ratios for better tolerance.
- Don’t ignore persistent metallic or bitter tastes—this may indicate excessive potassium or poor formulation.
- Don’t rely solely on taste to judge effectiveness—some key electrolytes like magnesium are poorly tasted but still essential.
Frequently Asked Questions
Can you taste all electrolytes?
No. While sodium and chloride produce clear salty tastes, other electrolytes like magnesium and calcium are either bitter or nearly tasteless. Potassium has a weak salty note but often brings bitterness due to activation of bitter taste receptors.
Why do some electrolyte drinks taste metallic?
A metallic aftertaste usually comes from potassium salts or certain forms of magnesium. It can also result from interactions between minerals and packaging materials, especially in cheaper formulations.
Is it safe to adjust the concentration of electrolyte drinks?
Yes, within reason. Diluting a solution can improve taste, but avoid extreme dilution, which reduces efficacy. Conversely, over-concentrating can lead to gastrointestinal discomfort or excessive sodium intake.
Practical Checklist: Optimizing Electrolyte Intake and Taste
- Choose electrolyte products with sodium as the primary cation for best taste and absorption.
- Look for added citric acid or natural fruit flavors to offset saltiness.
- Start with lower doses to allow your palate to adapt.
- Monitor how your body responds—cravings, thirst, and energy levels are clues.
- Store powders and tablets in airtight containers away from moisture to preserve flavor stability.
Conclusion: Embracing the Science of Salty Taste
The salty taste of electrolytes is far more than a quirk of flavor—it’s a biological signal rooted in necessity. From the ion channels on your tongue to the ancient instincts driving your cravings, every aspect of that taste serves a purpose. Recognizing this helps demystify why hydration doesn’t always come in sweet or neutral forms. Sometimes, the most effective solutions are the ones that challenge our taste buds, reminding us that health isn’t always about pleasure—but balance.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?