If you've ever cracked open a shaken can of beer only for it to erupt like a foam volcano, you're not alone. And if someone suggested tapping the can before opening—only to watch it still explode—the mystery deepens. What’s happening inside that aluminum cylinder? Why does tapping sometimes seem to help, and other times fail completely? The answer lies in fluid dynamics, nucleation, and the delicate balance of pressure and dissolved gas. This isn’t just party lore—it’s physics in action.
The Science of Carbonation: Trapped Gas Under Pressure
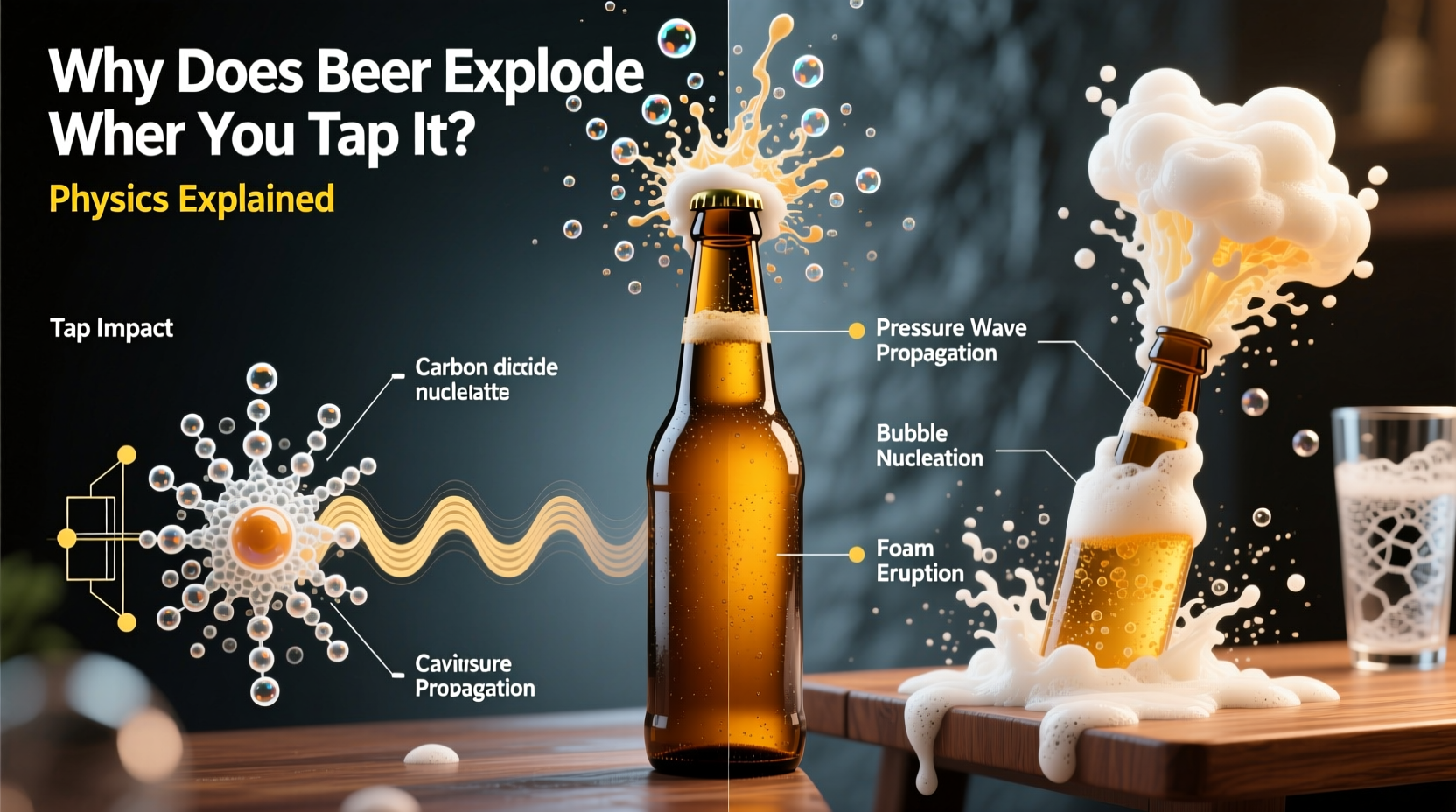
Beer is carbonated by dissolving carbon dioxide (CO₂) into liquid under high pressure. Inside a sealed can or bottle, the system is in equilibrium: CO₂ molecules are constantly moving between the gas space above the liquid (the headspace) and the beer itself. When sealed, pressure keeps most of the gas dissolved. But once the seal breaks, that balance collapses.
The solubility of CO₂ depends on both pressure and temperature. Cold beer holds more gas; warm beer releases it faster. Shake a can, and you disturb this stability. Tiny bubbles form along imperfections in the metal—scratches, dents, or microscopic pits—where gas can gather. These are called nucleation sites. Once formed, these bubbles grow rapidly when the can is opened due to sudden pressure drop.
What Happens When You Shake a Beer Can?
Shaking doesn’t add more gas—it redistributes existing CO₂. Mechanical agitation forces tiny bubbles to break free from the walls and disperse throughout the liquid. These suspended microbubbles act as ready-made launchpads for rapid expansion when pressure drops at opening.
Imagine each bubble as a seed for a larger one. When the can opens, atmospheric pressure plummets from around 30 psi inside to 14.7 psi outside. This sudden change causes every tiny bubble to expand explosively, pushing beer upward in a frothy jet. The result? A sticky mess and wasted drink.
Tapping Myth vs. Reality: Does It Really Work?
Tapping the bottom or sides of a shaken beer can has long been touted as a fix. The idea is that tapping dislodges bubbles clinging to the inner walls, allowing them to float to the top where they can safely join the headspace without causing eruption. In theory, fewer suspended bubbles mean less explosive foaming.
But here's the catch: **tapping only works if done correctly and immediately after shaking stops**. If too much time passes, bubbles have already dispersed through the liquid and begun coalescing. Tapping then does little. Moreover, aggressive tapping might even create new nucleation points, worsening the problem.
“Tapping reduces foam only when applied right after agitation, giving bubbles time to rise before opening.” — Dr. Antonio Vicente, Colloid Scientist, University of Birmingham
Step-by-Step Guide to Safely Open a Shaken Beer
Want to avoid the spray? Follow this science-based sequence:
- Stop shaking immediately. Let the can rest upright. Movement spreads bubbles; stillness lets them rise.
- Wait 30–60 seconds. This allows buoyant microbubbles to migrate to the surface and merge into the headspace.
- Gently tap the middle and bottom. Use fingertips or knuckles to encourage wall-bound bubbles to detach and rise.
- Open slowly. Crack the tab gradually instead of flipping it fast. This controls the rate of depressurization.
- Hold at an angle. Pour into a tilted glass to minimize turbulence and preserve carbonation.
Do’s and Don’ts of Handling Carbonated Beverages
| Action | Do | Don't |
|---|---|---|
| After shaking | Let sit upright for 1 minute | Open immediately |
| Tapping method | Light taps on side and base | Hard slaps or shaking upside down |
| Temperature | Keep cold until serving | Leave in hot car or direct sun |
| Opening technique | Slow, controlled release | Rapid full pull of tab |
| Pouring | Angle glass 45°, pour down side | Drop stream straight into center |
Mini Case Study: The Office Party Incident
Last summer, Mark brought six cans of IPA to a team celebration. While rushing from his car, he dropped the cooler. Later, eager to celebrate, he handed out the beers without checking their condition. One colleague opened hers—and within seconds, foam shot two feet into the air, soaking papers and keyboards.
Mark remembered hearing about tapping. He took the next can, tapped it gently five times on the bottom, waited half a minute, and opened it slowly. This time, only mild fizz escaped. The difference? Controlled bubble migration. By applying basic physics, he avoided further disaster and saved the remaining drinks.
Why Some Beers Foam More Than Others
Not all beers behave the same under stress. Factors influencing foam intensity include:
- Carbonation level: Highly carbonated lagers or pilsners tend to foam more than stouts or low-carb brews.
- Protein content: Beers rich in barley proteins stabilize foam, making eruptions thicker and longer-lasting.
- Presence of nitrogen: Some stouts use nitrogen instead of CO₂, which creates smaller, more stable bubbles and resists violent foaming.
- Can design: Modern \"anti-gushing\" cans feature smoother interiors to reduce nucleation sites.
Even additives like salt residues or lipstick on a bottle rim can trigger foaming. That’s why bartenders rinse glasses before pouring—to eliminate contaminants that promote bubble formation.
Frequently Asked Questions
Does tapping work on bottles as well as cans?
Tapping is less effective on glass bottles because their smooth interior offers fewer nucleation sites. However, letting a shaken bottle rest upright for a minute remains the best prevention strategy. Avoid wiping the neck or introducing foreign particles before opening.
Can you “un-shake” a beer?
No—but you can let it recover. Given enough time (typically 1–2 minutes), bubbles will naturally rise to the top and rejoin the headspace. Patience is more effective than any physical trick. There’s no way to reverse the kinetic energy introduced by shaking, but time restores equilibrium.
Is there a way to test if a beer is safe to open?
There’s no foolproof test without opening it. However, if you hear vigorous bubbling when gently tilting the can, it’s likely still agitated. A quiet can suggests the liquid has settled. Combine this with waiting and gentle tapping for best results.
Final Thoughts: Respect the Physics, Save the Beer
The next time you reach for a cold one after a bumpy ride or an accidental drop, remember: you’re not fighting magic or bad luck—you’re navigating the laws of physics. Dissolved gases, pressure gradients, and microscopic imperfections all play a role in whether your beer ends up in your glass or on your shirt.
Understanding the science transforms guesswork into control. With proper handling, timely pauses, and a light touch, you can consistently enjoy your drink without the drama.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?