Most substances contract when they solidify. As temperature drops, their molecules slow down and pack more tightly together, increasing density. Water, however, defies this norm. When it freezes into ice, it expands—becoming less dense than its liquid form. This unusual behavior has profound consequences in nature, from shaping landscapes to supporting aquatic life. Understanding why ice expands requires a dive into molecular structure, hydrogen bonding, and the delicate balance of energy and space at the atomic level.
The Anomaly of Water: A Scientific Exception
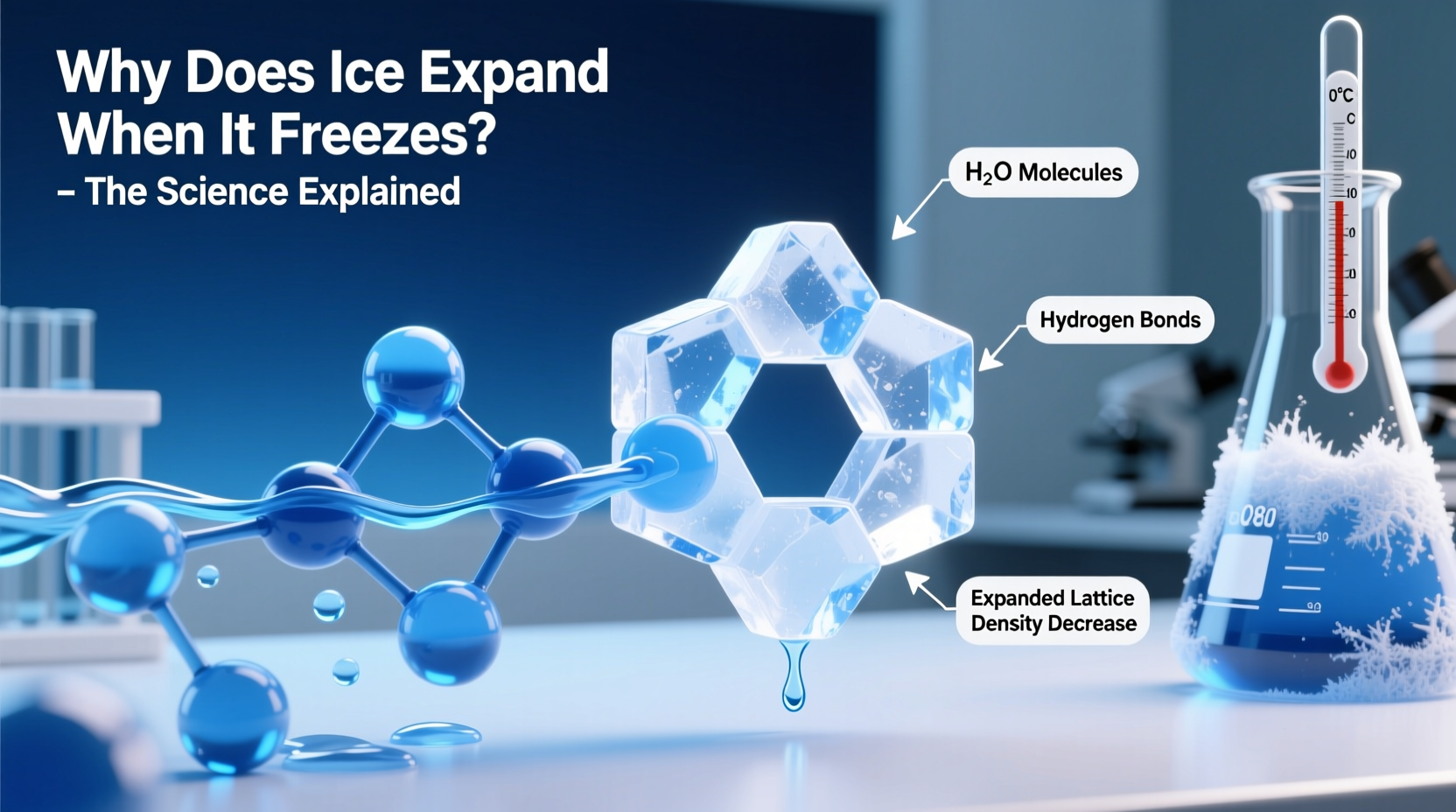
Water is one of the few substances on Earth that is less dense as a solid than as a liquid. This means that when water freezes, it takes up more volume than it did in liquid form. That’s why ice cubes float in a glass of water and why frozen pipes can burst under pressure.
This anomaly stems from the unique way water molecules interact with each other. In most liquids, cooling causes molecules to lose kinetic energy, move slower, and settle into a tightly packed solid structure. But in water, the formation of a crystalline lattice during freezing forces molecules into an open, hexagonal arrangement that actually creates more space between them.
Molecular Behavior: Hydrogen Bonds and the Hexagonal Lattice
The secret lies in hydrogen bonding. A water molecule (H₂O) consists of two hydrogen atoms covalently bonded to one oxygen atom. Oxygen is highly electronegative, meaning it pulls electrons toward itself, creating a partial negative charge on the oxygen and partial positive charges on the hydrogens.
This polarity allows water molecules to attract each other: the positive end of one molecule is drawn to the negative end of another, forming a hydrogen bond. These bonds are weaker than covalent bonds but strong enough to influence water’s physical properties significantly.
As water cools toward 4°C, the molecules slow down and begin to arrange themselves more efficiently, increasing density. But below 4°C, the directional nature of hydrogen bonds starts to dominate. Molecules orient themselves into a six-sided (hexagonal) crystal lattice—the foundation of ice. In this structure, each water molecule is hydrogen-bonded to four others, forming a rigid, open framework with large empty spaces in the center of each ring.
This ordered arrangement occupies more volume than the chaotic, closely packed molecules in liquid water. Hence, ice is about 9% less dense than liquid water at 4°C, causing it to expand by roughly the same percentage upon freezing.
“Water’s expansion upon freezing is not just a curiosity—it’s a cornerstone of Earth’s climate system and a prerequisite for life as we know it.” — Dr. Lena Patel, Physical Chemist, University of Colorado
Real-World Implications of Expanding Ice
The fact that ice floats may seem trivial, but it has massive ecological and geological consequences. If ice were denser than water, it would sink as it formed, leading to a chain reaction of freezing from the bottom up in lakes and oceans. Bodies of water would freeze solid over time, making aquatic life impossible in cold climates.
Instead, surface ice forms an insulating layer that slows further freezing, allowing liquid water—and life—to persist beneath. This principle supports ecosystems in polar regions and temperate zones alike.
On land, expanding ice drives mechanical weathering. Water seeps into cracks in rocks, freezes, expands, and exerts pressure—sometimes exceeding 2,000 atmospheres. Over time, this process breaks apart boulders and shapes mountain ranges, contributing to soil formation.
In human infrastructure, the same force causes challenges. Frozen water in concrete pores or metal pipes can lead to cracking, bursting, and costly damage—especially in regions with frequent freeze-thaw cycles.
Case Study: The Burst Pipe in a New England Winter
In January 2022, a homeowner in Vermont returned from vacation to find her basement flooded. Investigation revealed a ruptured copper pipe near an exterior wall. The cause? A drop in temperature caused stagnant water inside the pipe to freeze. As the ice expanded, it created immense pressure against the rigid metal walls. With no room to relieve the stress, the pipe fractured.
Though the repair cost exceeded $3,000, the incident highlighted a preventable issue rooted in basic physics. Insulating exposed pipes and maintaining indoor heat during cold spells could have avoided the disaster—all because of water’s tendency to expand when frozen.
Do’s and Don’ts: Managing Freezing Water in Practical Settings
| Action | Recommended? | Reason |
|---|---|---|
| Leave faucets dripping slightly in freezing weather | ✅ Yes | Flowing water is less likely to freeze; relieves pressure buildup |
| Insulate outdoor pipes with foam sleeves | ✅ Yes | Reduces heat loss and delays freezing |
| Use plastic containers for freezing liquids | ✅ Yes | Plastic can flex slightly under expansion stress |
| Fill glass jars completely before freezing | ❌ No | Glass is brittle and may shatter due to internal pressure |
| Turn off heating in unoccupied homes during winter | ❌ No | Risk of freezing and pipe bursts increases dramatically |
| Use antifreeze in car radiators | ✅ Yes | Lowers freezing point and prevents expansion damage |
Step-by-Step: How to Safely Freeze Water Without Damage
- Choose the right container: Use plastic or silicone vessels designed for freezing. Avoid sealed glass unless specifically labeled freezer-safe.
- Leave headspace: Fill containers only ¾ full to allow room for expansion.
- Freeze gradually: Place containers near the edge of the freezer first, then move toward the center to avoid thermal shock.
- Avoid overfilling bottles: For water bottles, leave at least 1–2 inches of air above the liquid line.
- Monitor thawing: Allow frozen items to thaw slowly in the refrigerator to minimize structural stress.
Frequently Asked Questions
Why doesn’t water behave like other liquids when it freezes?
Unlike most substances, water forms a stable, open hexagonal lattice due to hydrogen bonding. This structure has more space between molecules than in the liquid state, causing expansion instead of contraction.
At what temperature does water expand the most?
Water reaches its maximum density at approximately 4°C (39°F). As it cools further toward 0°C (32°F), it begins to expand. The greatest volume increase occurs precisely at the phase change—when liquid turns to solid ice.
Can you prevent water from expanding when it freezes?
Not entirely. While you can suppress freezing with pressure or additives (like salt or antifreeze), the fundamental expansion behavior remains inherent to pure water. Under extreme pressure, different forms of ice (like Ice III or Ice IX) can be denser than water, but these conditions don’t occur naturally on Earth’s surface.
Conclusion: Embracing the Quirk That Sustains Life
The expansion of ice is more than a scientific oddity—it’s a vital feature of our planet’s habitability. From protecting fish beneath winter lakes to carving mountains over millennia, this single property of water shapes environments and influences engineering practices worldwide. Recognizing the role of hydrogen bonding and molecular geometry helps demystify the phenomenon and underscores the elegance of natural laws.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?