Most substances contract when they cool and solidify. Water, however, defies this trend in one of the most consequential quirks of chemistry: it expands when it freezes. This seemingly simple behavior has profound implications for ecosystems, infrastructure, and even the evolution of life on Earth. Understanding why water behaves this way requires a dive into molecular structure, hydrogen bonding, and the delicate balance of energy states in matter.
The Anomalous Behavior of Water
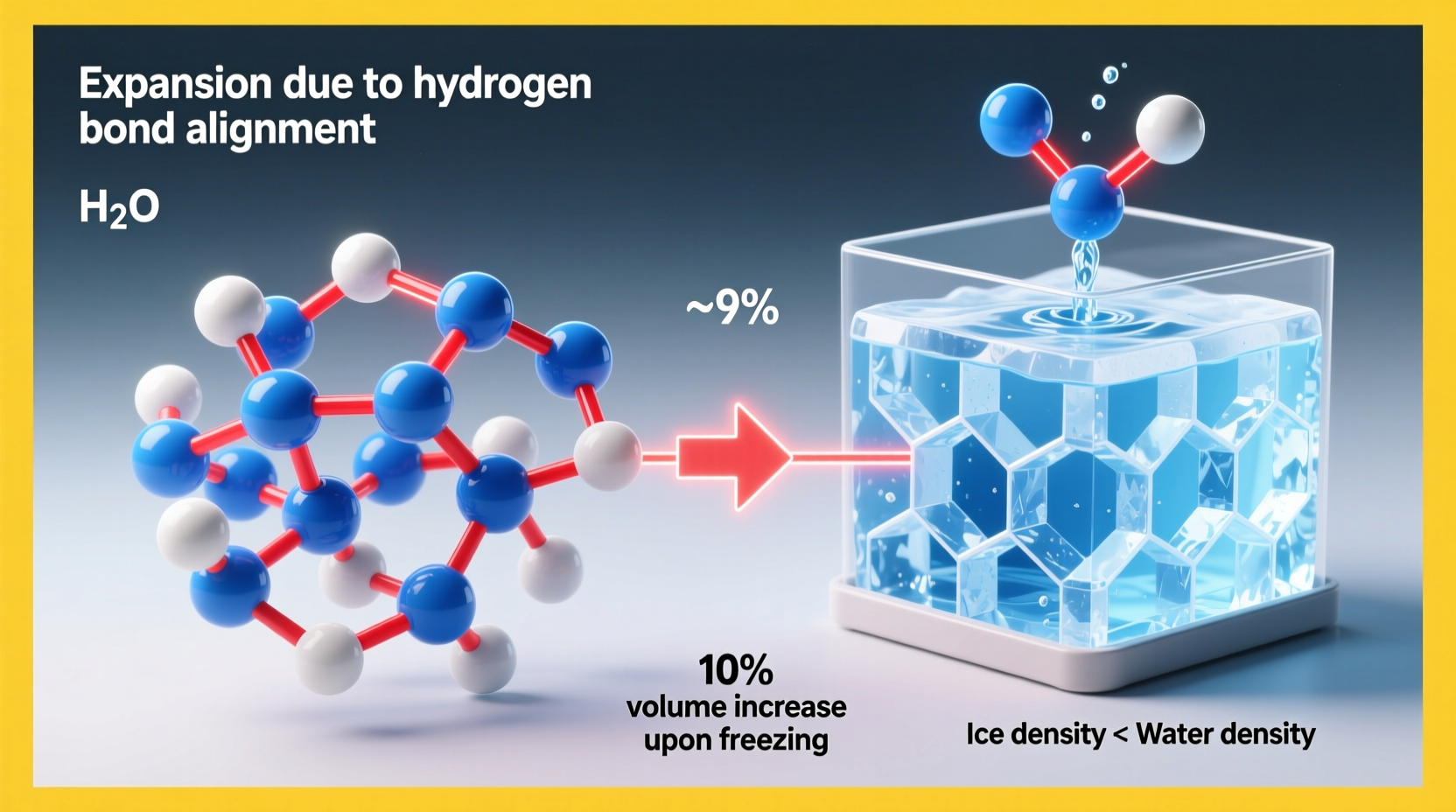
When most liquids transition from liquid to solid, their molecules slow down and pack more tightly, resulting in increased density. Water follows this pattern as it cools—but only down to 4°C (39°F). Below this temperature, something unusual happens: instead of continuing to contract, water begins to expand. At 0°C (32°F), when it freezes into ice, its volume increases by approximately 9%. This means that ice is less dense than liquid water, which is why ice cubes float in a glass and why lakes freeze from the top down rather than the bottom up.
This anomaly is not just a curiosity—it’s essential for life as we know it. If ice sank, bodies of water would freeze solid from the bottom, making aquatic life nearly impossible in cold climates. The fact that ice floats creates an insulating layer that protects organisms beneath.
Molecular Structure and Hydrogen Bonding
The root cause of water’s expansion lies in its molecular geometry and the powerful intermolecular forces known as hydrogen bonds. A water molecule (H₂O) consists of two hydrogen atoms bonded to one oxygen atom at an angle of about 104.5 degrees. This bent shape gives the molecule a polar character—oxygen carries a partial negative charge, while hydrogens carry partial positive charges.
These opposite charges allow water molecules to attract each other: the hydrogen of one molecule is drawn to the oxygen of another. This attraction forms a hydrogen bond—a relatively strong intermolecular force compared to others like van der Waals forces.
In liquid water, these bonds are constantly forming and breaking due to thermal motion. Molecules remain close but slide past one another freely. As water cools toward freezing, molecular movement slows, allowing hydrogen bonds to stabilize into a fixed, hexagonal lattice structure characteristic of ice.
The Hexagonal Lattice: Why Ice Takes Up More Space
When water freezes, every molecule forms four hydrogen bonds with neighboring molecules, creating a three-dimensional network shaped like a honeycomb. In this arrangement, molecules are held farther apart than they are in the liquid state. The open, rigid structure of ice contains large empty spaces between molecules, increasing its volume and decreasing its density.
Think of it like rearranging marbles in a box. In the liquid phase, marbles jostle and pack tightly. In the solid phase, they lock into a scaffold-like formation with gaps between them—taking up more space overall despite being made of the same material.
“Water’s expansion upon freezing is a textbook example of how molecular architecture dictates macroscopic behavior.” — Dr. Alan Reeves, Physical Chemist, MIT
Real-World Implications of Expanding Ice
The expansion of water during freezing isn’t just a scientific curiosity—it shapes landscapes, challenges engineers, and affects everyday homeowners. One of the most visible examples is frost weathering, where water seeps into cracks in rocks, freezes, expands, and exerts pressure strong enough to split stone over time. This process contributes significantly to mountain erosion and soil formation.
In urban environments, frozen water causes potholes. Rainwater enters small fissures in asphalt, freezes overnight, expands, and pushes the surrounding material apart. Repeated cycles widen the cracks until chunks of pavement break loose.
Mini Case Study: Burst Pipes in Winter
A homeowner in Minnesota neglected to insulate the basement pipes before winter. When temperatures dropped below freezing, the water inside the pipes began to turn to ice. As it expanded, pressure built rapidly—exceeding the tensile strength of the copper piping. Within hours, multiple joints ruptured, leading to flooding when the ice later thawed. The repair cost exceeded $5,000.
This scenario underscores a critical point: even a small volume of freezing water can generate enormous force. The expansion of water upon freezing can exert pressures exceeding 2,000 atmospheres under confined conditions—more than enough to crack metal or concrete.
Comparative Behavior of Liquids Upon Freezing
| Substance | Density Change on Freezing | Behavior |
|---|---|---|
| Water (H₂O) | Decreases (~9% increase in volume) | Expands; ice floats |
| Carbon dioxide (CO₂) | Increases | Contracts; dry ice sinks in liquid CO₂ |
| Acetone (C₃H₆O) | Increases | Contracts upon solidification |
| Mercury (Hg) | Increases | Contracts; solid mercury sinks |
| Silicon (Si) | Decreases | One of few elements that expands like water |
This table illustrates how rare water’s behavior is. Among common substances, only a handful—including silicon, gallium, germanium, and bismuth—expand upon freezing. But none have the environmental impact of water due to its abundance and role in biological systems.
Step-by-Step: How Water Transitions from Liquid to Solid
- Cooling Phase: Water temperature drops from room temperature toward 4°C. Density increases as molecules move slower and pack more closely.
- Anomaly Point: Below 4°C, hydrogen bonding starts to dominate over kinetic energy. Molecules begin aligning into tetrahedral arrangements.
- Nucleation: At 0°C, if a seed crystal (ice nucleus) is present, freezing begins. Molecules latch onto the growing ice lattice.
- Lattice Formation: Each water molecule forms four hydrogen bonds in a hexagonal structure, locking molecules into fixed positions with greater spacing.
- Expansion: As more molecules join the lattice, volume increases. Pressure may build if the water is confined.
- Stable Ice: Once fully frozen, the rigid, low-density structure remains stable until melting occurs.
Tips for Managing Water Expansion in Daily Life
- Never fill glass jars completely before placing them in the freezer.
- Use expansion tanks in closed-loop heating systems to absorb pressure from thermal expansion.
- Drain outdoor hoses and shut off exterior water valves in cold climates.
- Avoid pouring boiling water directly onto icy surfaces—rapid melting and refreezing can create slick hazards.
Frequently Asked Questions
Why doesn't water behave like other liquids when it freezes?
Water’s unique behavior stems from its polar molecular structure and strong hydrogen bonding. These forces favor an open, hexagonal lattice in the solid phase, which occupies more volume than the disordered but denser liquid phase.
Can water be prevented from expanding when it freezes?
No—expansion is inherent to the phase change under normal atmospheric conditions. However, applying extreme pressure can suppress expansion and lead to different forms of ice (e.g., ice II, III, V), which are denser than liquid water.
Does saltwater expand when it freezes?
Yes, but less than freshwater. Salt lowers the freezing point and disrupts crystal formation, resulting in slushy, briny ice with lower structural integrity. Still, seawater ice is less dense than liquid seawater and floats.
Conclusion: Embracing Water’s Unique Nature
The expansion of water upon freezing is a remarkable exception to the rules of physics that govern most materials. Rooted in the elegant dance of hydrogen bonds and molecular geometry, this phenomenon supports life, sculpts continents, and demands respect in engineering design. By understanding the science behind it, we gain deeper appreciation for the natural world and better tools to protect our homes and infrastructure.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?