Liquor sits comfortably in freezers around the world without turning solid, a fact that surprises many who assume all liquids freeze under cold enough conditions. Yet, whether it’s vodka pulled straight from a sub-zero freezer or a bottle of whiskey left overnight in an ice bath, most distilled spirits remain liquid even when exposed to extreme cold. The answer lies not in magic but in chemistry—specifically, in the freezing point depression caused by alcohol concentration. Understanding this phenomenon helps explain everything from cocktail preparation to proper storage and even homemade spirit production.
The Science Behind Freezing Points
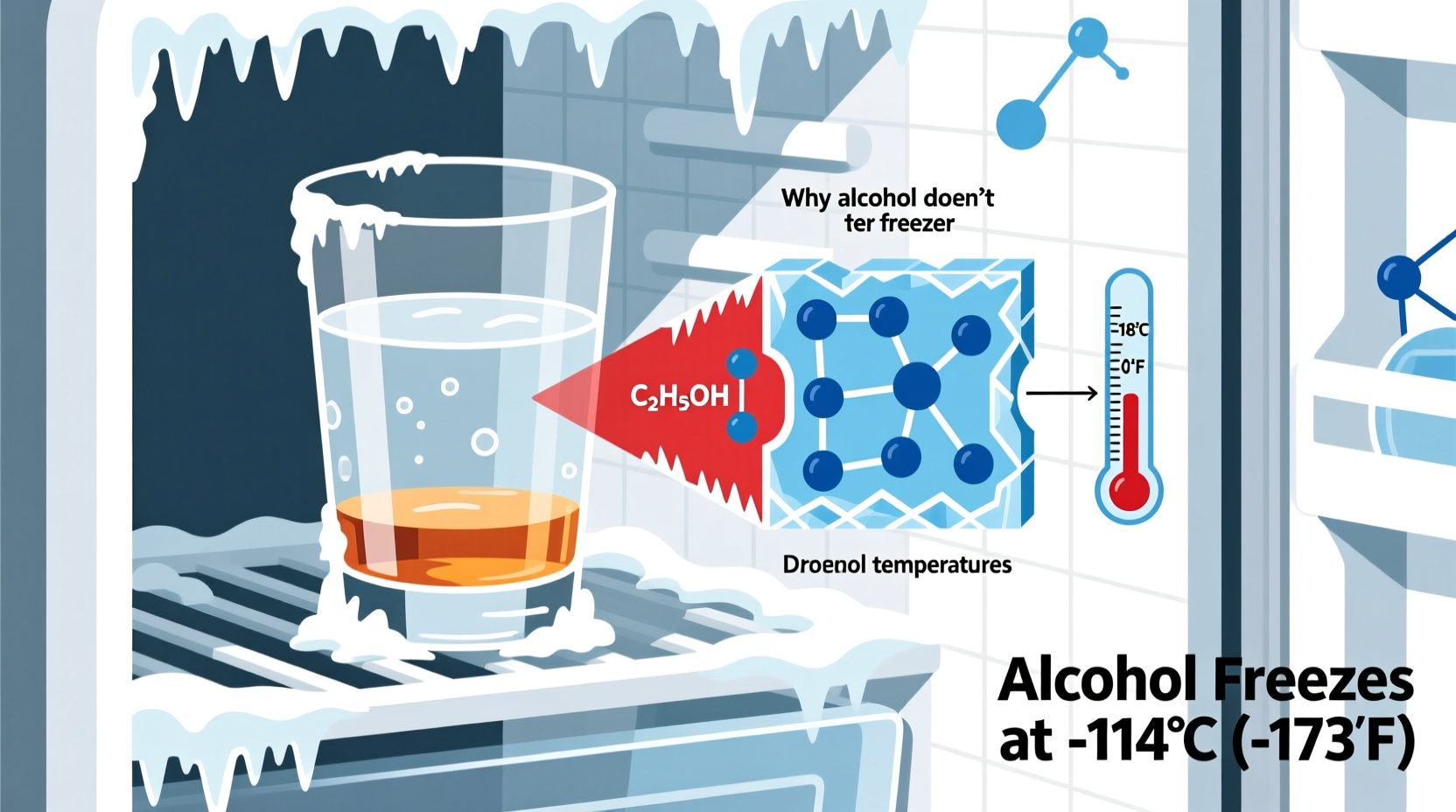
Water freezes at 0°C (32°F). This is common knowledge. But when other substances are dissolved into water, the freezing point drops—a principle known as freezing point depression. Ethanol, the type of alcohol found in alcoholic beverages, is fully miscible with water and significantly lowers the temperature at which the mixture solidifies.
As ethanol concentration increases, the freezing point continues to drop. Pure ethanol freezes at -114°C (-173°F), far below any household freezer’s capability. Most commercial liquors, however, are diluted with water, typically ranging from 40% to 60% alcohol by volume (ABV), which places their freezing thresholds well below typical freezer temperatures of -18°C (0°F).
This explains why your favorite bottle of rum or gin stays pourable after hours in the freezer: the alcohol content prevents complete crystallization.
Freezing Temperatures of Common Alcoholic Beverages
Different types of alcohol have varying ABV levels, which directly affect their freezing behavior. The table below outlines approximate freezing points for popular drinks based on standard concentrations.
| Drink Type | Alcohol by Volume (ABV) | Approximate Freezing Point |
|---|---|---|
| Beer | 4–6% | -2°C to -3°C (28°F to 27°F) |
| Wine | 12–14% | -5°C to -7°C (23°F to 19°F) |
| Vodka (80 proof) | 40% | -27°C (-16°F) |
| Rum (80 proof) | 40% | -27°C (-16°F) |
| Whiskey (86 proof) | 43% | -30°C (-22°F) |
| Gin (94 proof) | 47% | -34°C (-29°F) |
| Everclear (190 proof) | 95% | -110°C (-166°F) |
Note that while high-proof spirits like Everclear won’t freeze in any practical home environment, lower-alcohol drinks such as beer or wine will begin forming ice crystals if stored too long in a standard freezer—sometimes within just one hour.
How Alcohol Concentration Affects Freezing Behavior
The relationship between alcohol concentration and freezing point isn’t linear—it follows a curve where each additional percentage of ethanol has a diminishing effect on lowering the freezing point. At around 70% ABV, the mixture reaches its lowest possible freezing temperature before rising again due to changes in molecular interactions.
This non-linear behavior is critical for distillers and mixologists alike. For example, during fractional freezing (a method used in some traditional spirit-making processes), producers slowly cool fermented liquids so that water freezes first, leaving behind a more concentrated alcoholic solution when the ice is removed. While not as efficient as distillation, this technique leverages the differing freezing behaviors of water and ethanol.
“Ethanol disrupts the hydrogen bonding network of water, making it harder for ice crystals to form—even at very low temperatures.” — Dr. Alan Reyes, Physical Chemist, University of Colorado
Practical Applications: Storing and Chilling Liquor
Many people chill vodka, tequila, or liqueurs in the freezer for immediate use in cocktails. Because these spirits contain sufficient alcohol, they remain fluid and develop a smoother mouthfeel when served cold. However, there are nuances to consider:
- Flavor impact: Over-chilling can dull aromatic compounds in premium whiskeys or aged rums, reducing complexity.
- Bottle safety: Carbonated mixers or low-ABV liqueurs (like triple sec or amaretto) may partially freeze and expand, risking container rupture.
- Texture enhancement: Cream-based liqueurs (e.g., Irish cream) often separate or become slushy when frozen due to fat and sugar content.
If you're preparing drinks ahead of time, storing base spirits in the freezer ensures they’re ready to pour without needing ice—which would otherwise dilute the final product.
Step-by-Step Guide: Safely Chilling Spirits Without Damage
- Check the ABV: Only store spirits above 32% ABV (64 proof) in the freezer long-term.
- Screw cap tightly: Prevent evaporation and contamination by ensuring a secure seal.
- Avoid overfilling: Leave space in bottles containing mixtures with juice, dairy, or sugar.
- Limit duration: Even high-proof spirits should not stay in the freezer indefinitely; flavor stability matters.
- Label experimental bottles: If testing new infusions or homemade liqueurs, mark them clearly to avoid confusion.
Mini Case Study: The Home Bartender’s Freezer Experiment
Mark, an amateur mixologist in Minnesota, wanted to streamline his cocktail service during winter parties. He placed various bottles in his garage-mounted freezer, which runs at -23°C (-10°F). After 48 hours, he observed stark differences:
- Vodka (40% ABV): Fully liquid, slightly viscous—perfect for martinis.
- White Rum (37.5% ABV): Slightly thickened but still pourable.
- Coffee Liqueur (20% ABV): Partially frozen, with icy sludge forming at the bottom.
- Homemade Berry Infusion (28% ABV): Cloudy texture and sediment buildup after thawing.
From this experiment, Mark learned that only high-proof spirits could be reliably stored in his ultra-cold freezer. He now reserves the freezer for base alcohols and keeps lower-proof liqueurs refrigerated instead.
Common Myths About Alcohol and Freezing
Misconceptions abound when it comes to freezing alcohol. Here are two widely believed myths—and the truth behind them:
- Myth: “If it doesn’t freeze, it must not be real alcohol.”
Truth: This is false. Genuine alcohol resists freezing precisely because it *is* ethanol. Watered-down liquor would actually freeze more easily. - Myth: “Freezing removes alcohol content.”
Truth: No measurable evaporation occurs in sealed bottles at freezer temperatures. Alcohol content remains stable.
Frequently Asked Questions
Can any alcohol freeze in a home freezer?
Yes—low-alcohol beverages like beer, wine, and some liqueurs (especially cream-based ones) can partially or fully freeze in a standard freezer set to -18°C (0°F). Always check ABV before freezing.
Why does my bottle of whiskey look cloudy when chilled?
Cloudiness occurs when fatty acids, esters, or proteins in unfiltered spirits clump together at cold temperatures. This is normal and reversible upon warming. Many producers filter spirits at cold temperatures to prevent this in retail products.
Is it safe to drink frozen or slushy alcohol?
If only partially frozen and stored properly, yes. However, repeated freezing and thawing can alter texture and degrade flavor over time, especially in complex aged spirits.
Conclusion: Master the Cold, Respect the Chemistry
Understanding why liquor doesn’t freeze empowers smarter decisions—from cocktail prep to long-term storage. It’s not just trivia; it’s practical science that enhances both enjoyment and preservation of your favorite spirits. By respecting alcohol’s unique physical properties, you avoid ruined bottles, optimize chilling methods, and deepen your appreciation for the craftsmanship behind every pour.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?