In the world of chemistry and material science, identifying unknown substances is a fundamental task. Whether analyzing pollutants in water, verifying pharmaceutical purity, or determining the composition of a newly synthesized compound, scientists rely on measurable characteristics to make accurate identifications. Among these, intensive properties stand out as particularly powerful tools. Unlike measurements that depend on quantity, intensive properties remain consistent regardless of sample size—making them ideal for substance identification.
This article explains what intensive properties are, why they uniquely identify substances, and how they are used in real-world scientific analysis. We’ll also explore common examples, compare them with extensive properties, and demonstrate their practical value through case studies and expert insights.
Understanding Intensive vs. Extensive Properties
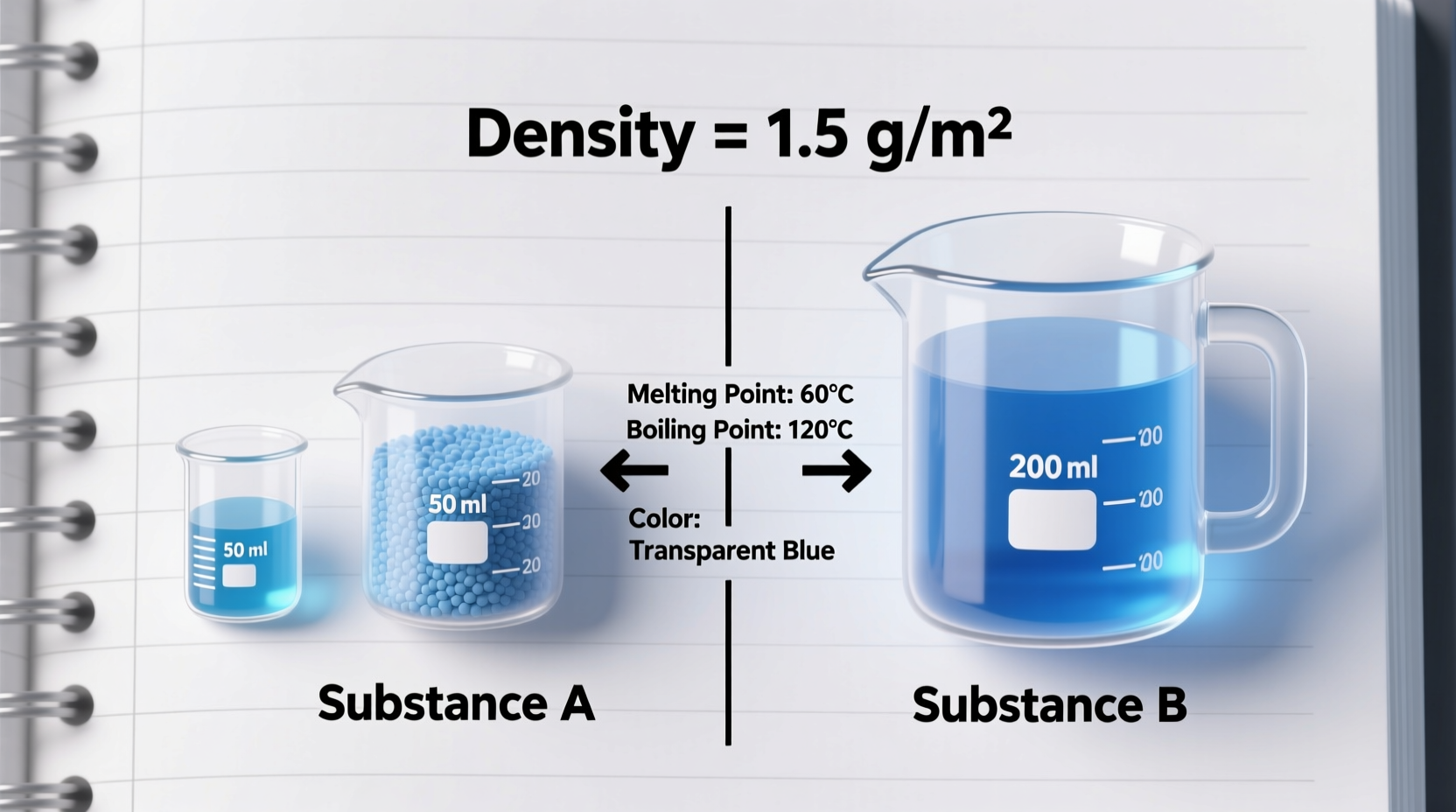
The distinction between intensive and extensive properties lies at the heart of physical science. An extensive property depends on the amount of matter present. Examples include mass, volume, length, and energy. If you double the amount of a substance, its mass and volume also double—these are extensive.
In contrast, an intensive property does not change with the quantity of the sample. It remains constant whether you have a gram or a kilogram of the material. These properties are inherent to the substance itself, not how much of it exists.
“Intensive properties act like fingerprints for materials. They allow us to classify and verify identity independent of scale.” — Dr. Lena Patel, Physical Chemist, MIT
For example, the density of gold is approximately 19.3 g/cm³, whether you’re holding a small flake or a solid bar. This consistency makes density—a classic intensive property—an excellent identifier.
Key Intensive Properties Used to Identify Substances
Several intensive properties are routinely used in laboratories and industrial settings to characterize and confirm the identity of substances. Below are the most important ones:
- Density: Mass per unit volume. Unique for pure substances under specific conditions.
- Melting Point: The temperature at which a solid becomes a liquid. Highly reproducible for pure compounds.
- Boiling Point: The temperature at which a liquid turns into vapor. Sensitive to molecular structure and intermolecular forces.
- Solubility: The ability of a substance to dissolve in a given solvent (e.g., water, ethanol). Often expressed as grams per 100 mL at a fixed temperature.
- Specific Heat Capacity: The amount of heat required to raise the temperature of one gram of a substance by one degree Celsius.
- Refractive Index: A measure of how light bends when passing through a substance. Used in optics and gemology.
- Electrical Conductivity: Ability to conduct electricity, crucial for distinguishing metals from nonmetals or semiconductors.
- Color and Odor: While subjective, these can serve as preliminary identifiers (e.g., chlorine’s greenish-yellow hue and pungent smell).
Why Intensive Properties Are Ideal for Identification
The reason intensive properties are so effective in identifying substances lies in their independence from sample size. Consider this scenario: a lab receives two samples—one weighing 5 grams and another 500 grams. If both exhibit the same melting point, boiling point, and density, it's strong evidence they are the same substance.
Because intensive properties reflect the internal structure and bonding of molecules or atoms, they are intrinsic to the chemical identity. Water (H₂O), for instance, will always boil at 100°C at standard atmospheric pressure, no matter the volume. This predictability allows scientists to build reference databases—such as the CRC Handbook of Chemistry and Physics—where known values are cataloged for comparison.
Moreover, impurities often alter intensive properties in detectable ways. For example, a melting point depression (lower than expected melting temperature) signals contamination. This sensitivity makes intensive properties useful not only for identification but also for assessing purity.
Practical Application: A Mini Case Study
A quality control technician at a pharmaceutical plant receives a white crystalline powder labeled “acetaminophen.” To verify its identity, she conducts three tests:
- Measures the melting point: observes it melts sharply at 169–171°C (matches literature value).
- Determines solubility: dissolves 14 g per 100 mL of water at 25°C (consistent with acetaminophen).
- Checks density: calculates 1.293 g/cm³ using displacement method (within acceptable range).
All results align with known intensive properties of pure acetaminophen. No additional structural analysis (like spectroscopy) is needed for initial verification. Had any value deviated significantly—say, a melting point of 150°C—the sample would be flagged for further testing, possibly indicating adulteration or mislabeling.
This case illustrates how intensive properties offer a fast, reliable, and cost-effective first line of defense in substance identification.
Comparison Table: Intensive vs. Extensive Properties
| Property Type | Depends on Amount? | Example | Useful for ID? |
|---|---|---|---|
| Intensive | No | Density of iron = 7.87 g/cm³ | Yes |
| Extensive | Yes | Mass = 25 g | No |
| Intensive | No | Boiling point of ethanol = 78.4°C | Yes |
| Extensive | Yes | Volume = 10 mL | No |
| Intensive | No | Color: sulfur is yellow | Limited (preliminary) |
Step-by-Step Guide to Identifying an Unknown Substance
Follow this systematic approach when attempting to identify an unknown sample using intensive properties:
- Observe Physical Characteristics: Note color, state (solid, liquid, gas), odor, and texture.
- Measure Density: Use mass and volume (via water displacement or dimensions) to calculate density.
- Determine Melting/Boiling Point: Use calibrated equipment; compare values to reference tables.
- Test Solubility: Try dissolving in water, alcohol, and oil. Record results at controlled temperatures.
- Compare with Known Data: Consult reliable sources like chemical handbooks or databases.
- Confirm with Advanced Methods (if needed): Use spectroscopy (IR, NMR) or chromatography for definitive confirmation.
Frequently Asked Questions
Can two different substances have the same intensive properties?
It’s rare but possible for two substances to share one or two intensive properties (e.g., similar densities). However, the likelihood of multiple intensive properties matching—such as identical melting point, boiling point, and solubility—is extremely low. Scientists use a combination of properties to minimize misidentification.
Is temperature an intensive property?
Yes, temperature is intensive. A cup of boiling water and a pot of boiling water both have a temperature of 100°C at sea level, regardless of volume. Temperature measures average kinetic energy per molecule, not total energy.
Why can’t we use mass or volume alone to identify a substance?
Mass and volume are extensive properties—they change with sample size. A large piece of aluminum has more mass than a small piece of gold, even though gold is denser. Without normalizing for size (as density does), these measurements cannot reliably indicate identity.
Checklist: Verifying a Substance Using Intensive Properties
- ☑ Observe appearance and physical state
- ☑ Measure mass and volume to calculate density
- ☑ Determine melting point (for solids) or boiling point (for liquids)
- ☑ Test solubility in common solvents
- ☑ Compare all data with authoritative chemical references
- ☑ Repeat tests for consistency
- ☑ Use instrumental analysis if uncertainty remains
Conclusion
Intensive properties are indispensable in the scientific toolkit for identifying substances. Their independence from quantity allows for consistent, scalable analysis across fields—from forensic science to pharmaceutical manufacturing. By relying on intrinsic characteristics like density, melting point, and solubility, researchers can quickly and confidently determine what a material is, even with minimal sample sizes.
Mastering the use of intensive properties isn't just about memorizing numbers—it's about understanding the deeper connection between a substance’s behavior and its molecular identity. As analytical techniques evolve, these foundational principles remain unchanged, proving their enduring value in science.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?