Metallic materials are foundational to modern engineering, from construction beams to aerospace components. Among the various types of alloys, interstitial alloys stand out due to their unique atomic structure and mechanical properties. One notable characteristic is their reduced malleability compared to pure metals or substitutional alloys. Understanding why interstitial alloys are less malleable requires a deep dive into atomic interactions, lattice distortions, and dislocation dynamics. This article explains the science behind this phenomenon, supported by real examples, expert insights, and practical implications for material selection.
What Are Interstitial Alloys?
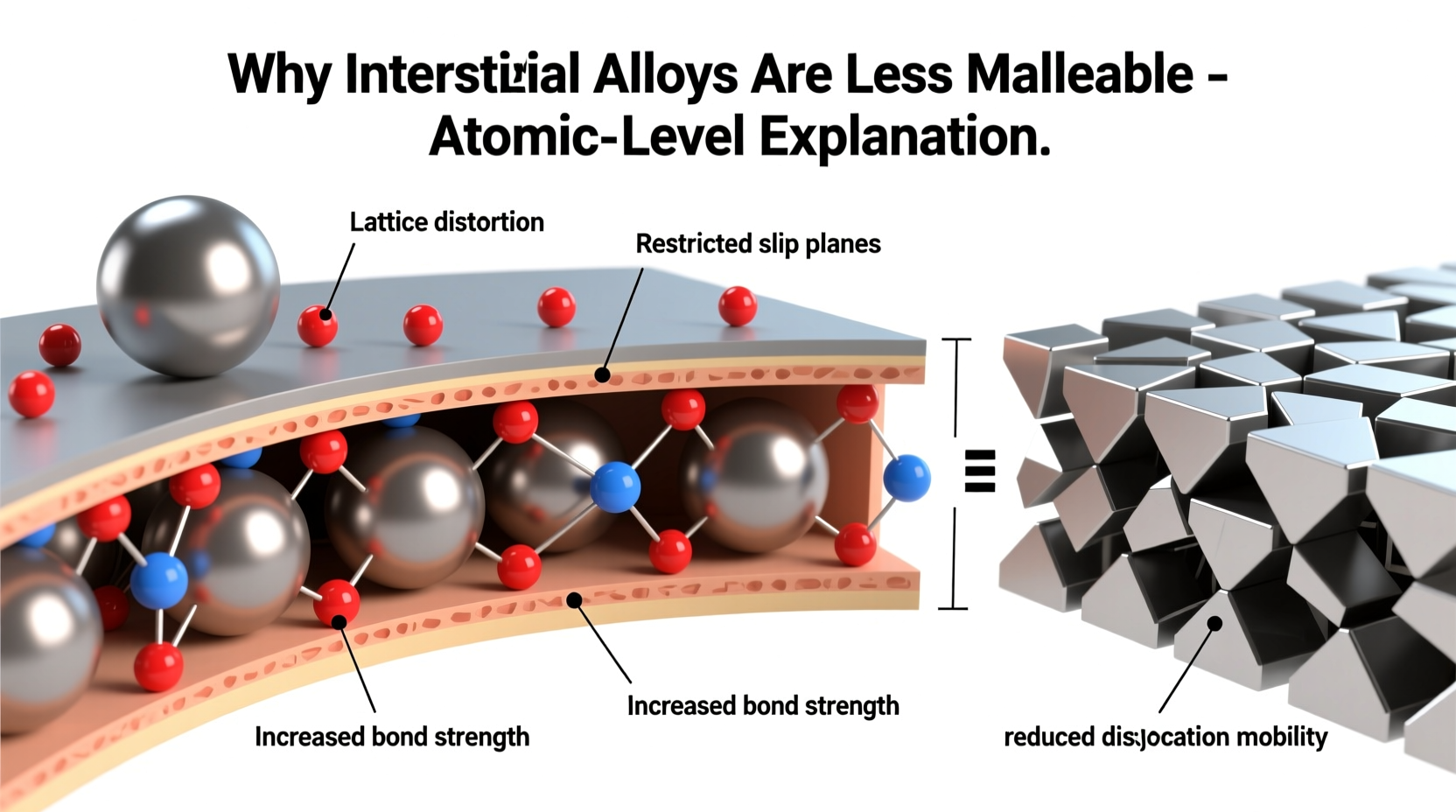
Interstitial alloys form when atoms of a smaller element occupy the spaces—called interstices—between the atoms of a larger host metal in its crystal lattice. Common examples include carbon in iron (forming steel), nitrogen in titanium, or hydrogen in palladium. The guest atoms do not replace the host atoms but instead fit into the gaps within the metallic structure.
This structural arrangement fundamentally alters the physical behavior of the base metal. While these alloys often gain strength and hardness, they typically lose some ductility and malleability—the ability to deform under stress without fracturing.
Atomic Distortion and Lattice Strain
The primary reason interstitial alloys are less malleable lies in the distortion they introduce into the crystal lattice. When small atoms like carbon enter the interstitial sites of a metal such as iron, they push neighboring atoms slightly apart. This creates localized strain fields that disrupt the regular, repeating pattern of the lattice.
This strain interferes with the movement of dislocations—line defects in the crystal structure that allow metals to deform plastically. In pure metals, dislocations can glide easily across planes when force is applied, enabling bending or shaping (malleability). But in interstitial alloys, the distorted regions act as obstacles, pinning dislocations in place.
The stronger the interaction between the interstitial atom and the surrounding lattice, the greater the resistance to dislocation motion. For example, in steel, each carbon atom exerts a significant elastic strain on the iron lattice, making it harder for layers of atoms to slide past one another.
“Adding even 0.2% carbon to iron transforms it from a soft, malleable metal into a rigid structural material. That’s the power of interstitial strengthening.” — Dr. Alan Reyes, Materials Scientist at MIT
Dislocation Pinning and Work Hardening
Dislocation movement is essential for plastic deformation. Malleability depends on how freely these dislocations can travel through the crystal. In interstitial alloys, the foreign atoms serve as pinning points.
Imagine trying to move a rug across a floor littered with small stones. The stones resist smooth sliding, just as interstitial atoms resist dislocation glide. This phenomenon is known as solute strengthening or interstitial solid solution strengthening.
Moreover, once deformation begins, dislocations multiply and tangle around these obstacles, increasing internal stress. This leads to work hardening—the material becomes stronger but more brittle with continued deformation. As a result, interstitial alloys often crack before undergoing significant shape change.
Step-by-Step: How Interstitial Alloying Reduces Malleability
- Alloy Formation: Small atoms (e.g., C, N, H) diffuse into the crystal lattice of a host metal (e.g., Fe, Ti).
- Lattice Distortion: The interstitial atoms push adjacent metal atoms out of position, creating strain.
- Dislocation Interaction: Moving dislocations encounter these strained zones and become pinned.
- Reduced Mobility: Dislocation glide is hindered, requiring higher stress to continue deformation.
- Loss of Malleability: The metal resists bending and shaping, becoming harder but less ductile.
Comparison: Substitutional vs. Interstitial Alloys
| Feature | Substitutional Alloys | Interstitial Alloys |
|---|---|---|
| Atom Placement | Foreign atoms replace host atoms | Foreign atoms occupy spaces between host atoms |
| Size Requirement | Similar atomic radii | Solute much smaller than solvent |
| Example | Brass (Zinc in Copper) | Steel (Carbon in Iron) |
| Lattice Distortion | Moderate | High (localized strain) |
| Effect on Malleability | Slight reduction | Significant reduction |
| Primary Strengthening Mechanism | Lattice mismatch scattering | Dislocation pinning via strain fields |
Real-World Example: Steel vs. Pure Iron
A practical illustration of reduced malleability in interstitial alloys can be seen in the comparison between pure iron and low-carbon steel.
Pure iron is relatively soft and highly malleable. It can be rolled into thin sheets or drawn into wires with minimal cracking. However, it lacks strength for structural applications. When carbon (typically 0.05–0.25%) is added interstitially, forming mild steel, the material gains tensile strength and hardness. But this comes at a cost: the same steel cannot be bent as sharply as pure iron without risk of fracture.
In manufacturing, this trade-off is critical. For instance, in automotive body panels, engineers select specific grades of steel based on the required balance between formability (malleability) and strength. High-strength low-alloy (HSLA) steels use controlled interstitial content to optimize performance without sacrificing too much ductility.
Expert Strategies to Manage Malleability in Alloy Design
Metallurgists use several techniques to mitigate excessive loss of malleability while still benefiting from interstitial strengthening:
- Controlled Carbon Content: Limiting carbon to below 0.3% preserves some ductility in steel.
- Heat Treatment: Processes like annealing relieve internal stresses and restore limited malleability after cold working.
- Grain Refinement: Smaller grains improve toughness and can offset some brittleness caused by interstitials.
- Use of Stabilizing Elements: Adding elements like titanium or niobium can bind excess carbon, reducing free interstitials.
Frequently Asked Questions
Why doesn’t adding more carbon always make steel better?
While carbon increases strength and hardness, excessive amounts (above 0.8%) drastically reduce ductility and weldability. High-carbon steels become brittle and prone to cracking during shaping or under impact, limiting their use in many structural applications.
Can interstitial alloys ever be malleable?
To a limited extent. Very low concentrations of interstitial atoms (e.g., ultra-low-carbon steel) retain reasonable malleability. Additionally, thermomechanical processing such as hot rolling or annealing can restore some ductility by reorganizing the microstructure and redistributing interstitial atoms.
Are all small atoms used in interstitial alloys?
No. Only certain elements—primarily carbon, nitrogen, hydrogen, and sometimes boron—form true interstitial solutions. Their atomic size must be small enough to fit into tetrahedral or octahedral voids in the host lattice without causing catastrophic disruption.
Conclusion: Balancing Strength and Malleability
The reduced malleability of interstitial alloys is not a flaw but a consequence of deliberate design. By introducing small atoms into a metal’s lattice, engineers enhance strength at the expense of formability. This trade-off is central to materials science and enables the creation of high-performance alloys used in bridges, vehicles, tools, and machinery.
Understanding the atomic-level mechanisms—lattice strain, dislocation pinning, and work hardening—empowers better decision-making in material selection and processing. Whether you're designing a new component or selecting stock for fabrication, recognizing how interstitial elements affect behavior ensures optimal performance and longevity.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?