Electricity is the flow of charged particles. In metals, this flow occurs through free-moving electrons. But in ionic compounds, conduction works differently—only under certain conditions. Understanding why ionic compounds conduct electricity requires a closer look at their structure, bonding, and behavior in different states. This article breaks down the science clearly, explains when and how these compounds conduct, and explores real-world implications.
The Structure of Ionic Compounds
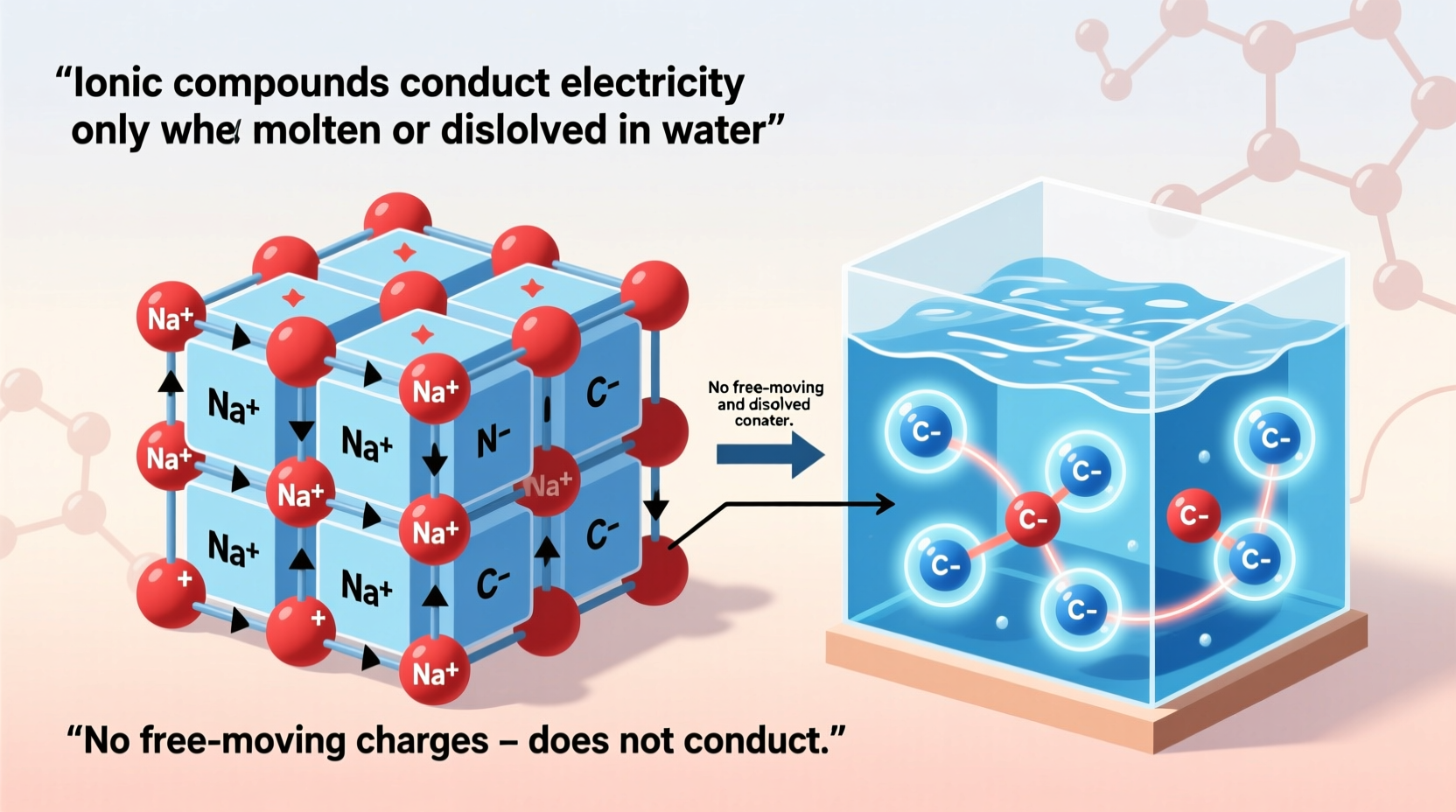
Ionic compounds are formed when atoms transfer electrons to create positively charged cations and negatively charged anions. These ions are held together by strong electrostatic forces in a rigid, repeating lattice structure. Common examples include sodium chloride (NaCl), potassium iodide (KI), and calcium fluoride (CaF₂).
In the solid state, the ions are locked in place within the crystal lattice. Although they carry charge, they cannot move freely. Because electrical conduction requires the movement of charged particles, solid ionic compounds do not conduct electricity despite being made of ions.
Why Molten Ionic Compounds Conduct Electricity
When an ionic compound is heated to its melting point, the rigid lattice breaks down. The ions are no longer fixed in position and gain enough kinetic energy to move freely. In this molten (liquid) state, the positive and negative ions can migrate toward oppositely charged electrodes when a voltage is applied.
For example, in molten sodium chloride:
- Na⁺ ions move toward the negative electrode (cathode), where they gain electrons and form sodium metal.
- Cl⁻ ions move toward the positive electrode (anode), where they lose electrons and form chlorine gas.
This movement of ions constitutes an electric current. Therefore, molten ionic compounds are good conductors of electricity due to mobile ions.
Conduction in Aqueous Solutions
When ionic compounds dissolve in water, they dissociate into their constituent ions. Water molecules, being polar, surround the ions and stabilize them in solution—a process known as hydration. For instance, NaCl dissolves to form Na⁺(aq) and Cl⁻(aq) ions dispersed throughout the water.
These hydrated ions are free to move and respond to an electric field. When electrodes are placed in the solution, cations drift toward the cathode and anions toward the anode, enabling the flow of current. This is why saltwater conducts electricity, while pure water (with very few ions) does not.
“Electrolytic conduction in solutions depends entirely on the presence of mobile ions. Without ion mobility, there is no current.” — Dr. Alan Reyes, Physical Chemist
Key Conditions for Electrical Conduction
Not all forms of ionic compounds conduct electricity. The ability to conduct depends on one critical factor: ion mobility. Below is a summary of conduction across different states.
| State | Ion Mobility | Conducts Electricity? | Explanation |
|---|---|---|---|
| Solid | Fixed in lattice | No | Ions cannot move; no charge flow |
| Molten (Liquid) | Mobile | Yes | Heat breaks lattice; ions move freely |
| Aqueous (Dissolved in Water) | Hydrated and mobile | Yes | Water separates ions; solution allows migration |
Step-by-Step: Testing Ionic Conductivity
To observe whether an ionic compound conducts electricity, follow this simple lab procedure:
- Prepare the sample: Choose a pure ionic compound like NaCl.
- Set up the circuit: Use a power source, wires, electrodes (e.g., graphite rods), and a bulb or ammeter in series.
- Test in solid state: Place solid NaCl between electrodes. Observe no current (bulb remains off).
- Heat to melt: Melt the compound using a Bunsen burner. The bulb lights up, indicating conduction.
- Dissolve in water: Repeat with NaCl dissolved in distilled water. Bulb lights again, confirming aqueous conduction.
- Compare results: Note that conduction only occurs when ions are mobile.
Real-World Example: Electrolysis of Brine
A practical application of ionic conduction is the electrolysis of brine (concentrated sodium chloride solution). In industrial settings, this process produces chlorine gas, hydrogen gas, and sodium hydroxide—all valuable chemicals.
When a direct current is passed through the solution:
- Chloride ions (Cl⁻) are oxidized at the anode to form Cl₂ gas.
- Water molecules are reduced at the cathode to produce H₂ gas and OH⁻ ions.
- Sodium ions (Na⁺) remain in solution, combining with OH⁻ to form NaOH.
This reaction only works because the ions in the solution are free to move and participate in redox reactions at the electrodes. It’s a clear demonstration of how ionic conductivity enables large-scale chemical production.
Common Misconceptions Clarified
Many people assume that all compounds containing ions automatically conduct electricity. This is not true. The key is not just the presence of ions, but their ability to move. Here are some frequent misunderstandings:
- Misconception: “All salts conduct electricity.” Reality: Only when molten or dissolved. Solid table salt won’t complete a circuit.
- Misconception: “Ionic compounds conduct like metals.” Reality: Metals use electron flow; ionic compounds rely on ion migration, which is slower and involves chemical change.
- Misconception: “Sugar water conducts because it dissolves.” Reality: Sugar is covalent and doesn’t form ions, so it doesn’t conduct—even when dissolved.
Frequently Asked Questions
Can ionic compounds conduct electricity in the solid state if they have defects?
Minor defects in the crystal lattice may allow slight ion movement at very high temperatures, leading to minimal conductivity. However, this is negligible compared to molten or aqueous states and not sufficient for practical conduction.
Why don’t all ionic compounds dissolve in water?
Solubility depends on the balance between the lattice energy (holding ions together) and hydration energy (released when ions interact with water). If lattice energy is too high, the compound won’t dissolve—like calcium carbonate (CaCO₃)—and thus won’t conduct in water unless melted.
Is the conductivity of ionic solutions proportional to concentration?
Up to a point, yes. Higher concentrations mean more ions available to carry charge. However, at very high concentrations, ion pairing and reduced mobility can decrease conductivity. There’s an optimal concentration for maximum conduction.
Checklist: Assessing Ionic Conductivity
Use this checklist to determine whether an ionic compound will conduct electricity:
- ✅ Is the compound in a molten state? → Likely conducts
- ✅ Is it dissolved in a polar solvent like water? → Likely conducts
- ✅ Is it in solid form? → Does not conduct
- ✅ Are free-moving ions present? → Essential for conduction
- ✅ Is there an applied voltage? → Needed to drive ion migration
Conclusion
The ability of ionic compounds to conduct electricity hinges on one fundamental principle: ion mobility. Whether through melting or dissolution, the breakdown of the rigid lattice allows charged particles to move and carry current. This principle underpins technologies from batteries to industrial electrolysis and helps explain everyday phenomena like why ocean water conducts electricity but solid salt does not.
Understanding this concept goes beyond textbook knowledge—it empowers better decision-making in labs, industries, and even safety practices (e.g., avoiding electrical devices near saltwater). Now that you know the science behind ionic conductivity, consider how this knowledge applies in your environment.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?