Air surrounds us constantly, yet few pause to consider its true composition. It's easy to assume that the invisible substance we breathe is a single, uniform entity. However, air is not a pure substance or a chemical compound—it is a mixture. This distinction is crucial in chemistry and atmospheric science. Understanding why air qualifies as a mixture rather than a compound involves examining its physical behavior, compositional variability, and response to separation techniques. Through scientific observation and experimentation, multiple lines of evidence confirm that air is a homogeneous mixture of gases, each retaining its individual properties.
What Defines a Mixture vs. a Compound?
Before delving into the nature of air, it’s essential to clarify the difference between a mixture and a compound. A compound forms when two or more elements chemically bond in fixed proportions, resulting in a new substance with unique properties. Water (H₂O), for example, is a compound—hydrogen and oxygen lose their original characteristics and form a stable molecule through chemical bonding.
In contrast, a mixture consists of two or more substances physically combined without forming new chemical bonds. The components retain their identities and can usually be separated by physical means. Mixtures can be homogeneous (uniform throughout) or heterogeneous (non-uniform). Air is a homogeneous mixture because its gases are evenly distributed on a molecular level under normal conditions.
Evidence That Air Is Not a Compound
If air were a compound, it would exhibit consistent behavior across all samples: fixed composition, definite melting and boiling points, and inseparability without chemical reactions. But none of these apply. Instead, air shows variable composition, lacks sharp phase transition points, and its components can be isolated using physical methods—all hallmarks of a mixture.
Key Evidence Supporting Air as a Mixture
1. Variable Composition of Gases
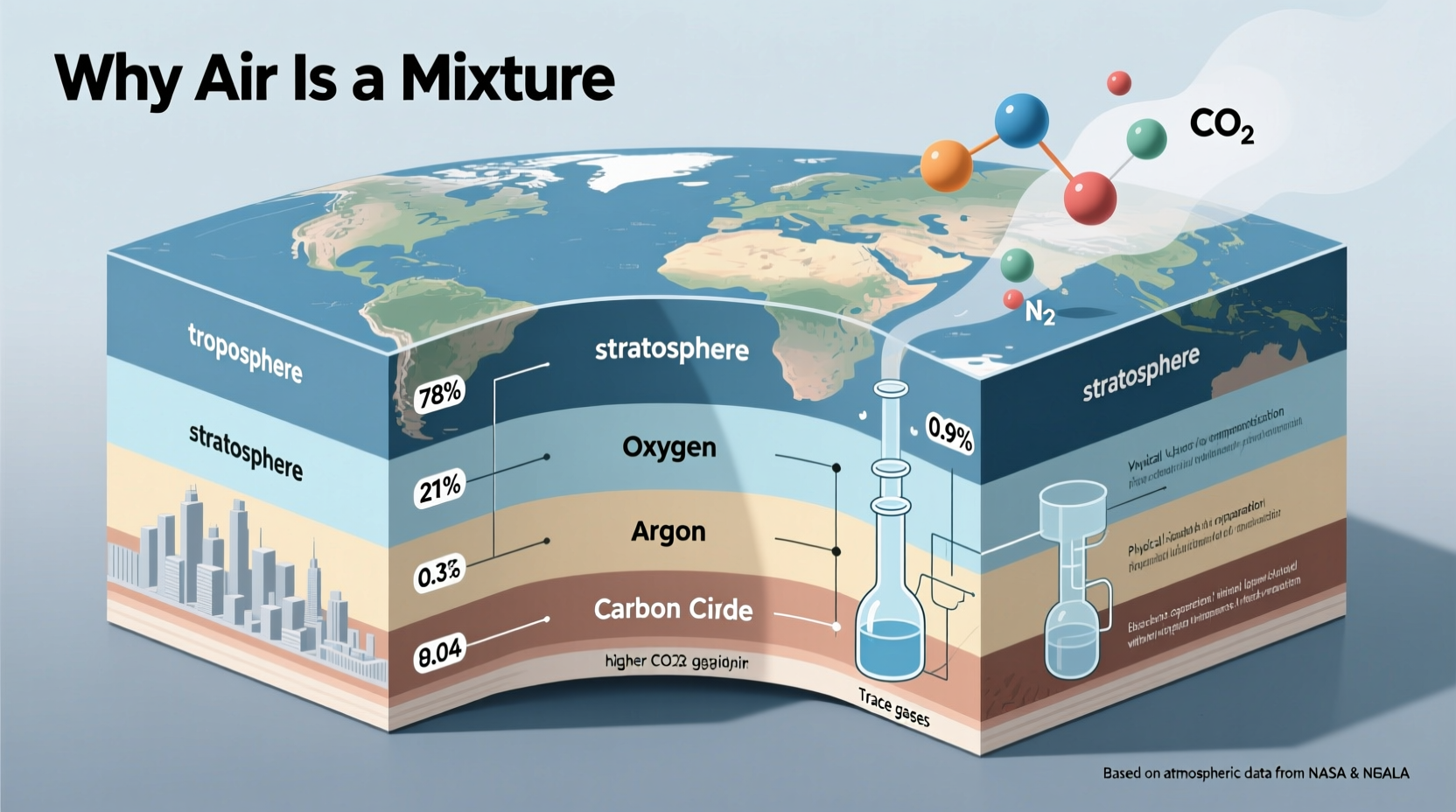
One of the strongest indicators that air is a mixture is the variability of its components. While nitrogen (~78%) and oxygen (~21%) dominate, their exact percentages fluctuate based on location, altitude, humidity, and pollution levels. For instance:
- Water vapor content can range from 0% to 4%, depending on climate.
- Carbon dioxide varies from 0.04% today to higher levels in urban areas.
- Noble gases like argon remain relatively constant, but trace pollutants such as methane or ozone appear only in certain regions.
This variability contradicts the defining feature of compounds, which must have a fixed ratio of elements. No chemical formula can represent air universally because its composition isn’t constant.
2. Physical Separation of Components
Gases in air can be separated without breaking chemical bonds, further proving its status as a mixture. Fractional distillation of liquefied air is a widely used industrial method where cooled air is gradually warmed, allowing gases to boil off at different temperatures:
| Gas | Boiling Point (°C) | Separation Stage |
|---|---|---|
| Nitrogen (N₂) | -196 | First to evaporate |
| Argon (Ar) | -186 | Middle stage |
| Oxygen (O₂) | -183 | Late stage |
The ability to separate gases via physical processes like distillation, diffusion, or selective absorption confirms they are not chemically bonded. In a true compound, such separation would require energy-intensive chemical decomposition.
3. Retention of Individual Properties
In air, each gas behaves independently. Oxygen supports combustion, nitrogen acts as an inert diluent, and carbon dioxide dissolves in water to form weak carbonic acid. These properties persist even within the mixture. For example, a glowing splint reignites in air due to oxygen’s presence—nitrogen doesn’t interfere with this chemical behavior. If air were a compound, such selective reactivity would not occur; the entire substance would exhibit unified chemical traits.
“Air’s components coexist without altering one another’s fundamental behaviors—a textbook characteristic of physical mixtures.” — Dr. Lena Patel, Atmospheric Chemist, University of Edinburgh
4. Lack of Fixed Melting and Boiling Points
Pure substances and compounds have sharp, defined melting and boiling points. Air, however, liquefies and vaporizes over a range of temperatures. As air is cooled, different gases condense at different stages rather than simultaneously. This gradual phase change is typical of mixtures and inconsistent with the behavior of compounds.
5. No Chemical Reaction Upon Mixing
The formation of a compound releases or absorbs significant energy (exothermic or endothermic reaction). When nitrogen and oxygen mix in the atmosphere, no such energy change occurs. Their combination is purely physical and reversible. Even under standard conditions, N₂ and O₂ do not spontaneously react to form nitrogen oxides—this requires high temperatures, such as in lightning strikes or combustion engines. The absence of inherent reactivity upon mixing reinforces that air is a mechanical blend, not a chemically bonded entity.
Real-World Example: Industrial Gas Production
A practical illustration of air’s nature as a mixture comes from cryogenic air separation plants. These facilities supply medical oxygen, industrial nitrogen, and argon for welding. The process begins by filtering and compressing ambient air, then cooling it below -200°C until it liquefies. Through fractional distillation in a distillation column, nitrogen boils off first, followed by argon, then oxygen. The entire operation relies on physical differences in volatility—no chemical reactions are involved.
This large-scale application wouldn't be feasible if air were a compound. Breaking chemical bonds to extract oxygen would consume far more energy and yield less purity. The economic viability of air separation hinges on air being a physical mixture.
Common Misconceptions About Air
Some mistakenly believe air is a compound because it appears uniform and stable. However, homogeneity does not imply chemical bonding. Saltwater is also homogeneous but remains a mixture. Similarly, the stability of air arises from the inertness of nitrogen and balanced atmospheric dynamics—not from chemical unity.
Another misconception is that since air has an \"average\" formula (often approximated as 78% N₂, 21% O₂, 1% Ar), it must be a compound. But this is merely a statistical summary, not a molecular formula. Compounds like CO₂ have identical molecules throughout; air contains billions of independent N₂, O₂, and other gas molecules moving freely.
Step-by-Step: How Scientists Prove Air Is a Mixture
- Collect air samples from various environments (urban, rural, high altitude).
- Analyze gas composition using gas chromatography or mass spectrometry.
- Observe variations in oxygen, CO₂, and moisture levels across samples.
- Cool air to liquid state and perform fractional distillation.
- Measure boiling points of evolving gases—distinct values confirm separate substances.
- Test individual gases post-separation for original chemical properties (e.g., O₂ reigniting a splint).
- Conclude that physical separability and retained properties confirm a mixture.
Frequently Asked Questions
Can air ever become a compound?
No. While some gases in air can react under extreme conditions (like NO formation during lightning), the bulk composition remains a physical mixture. The majority of nitrogen and oxygen do not chemically combine under normal atmospheric conditions.
Why doesn’t air settle into layers if it’s a mixture?
Although gases have different densities, constant molecular motion (Brownian motion) and atmospheric turbulence keep them well-mixed. Diffusion ensures homogeneity over time, especially near the Earth’s surface.
Is polluted air still considered a mixture?
Yes. Even with added pollutants like sulfur dioxide or particulate matter, air remains a mixture—now a more complex one. Pollutants disperse physically and can be filtered or precipitated out without altering the fundamental gaseous matrix.
Actionable Checklist: Understanding Air Composition
- ✅ Recognize that air has no fixed chemical formula.
- ✅ Remember that physical methods can separate its components.
- ✅ Observe how gas properties remain unchanged in air.
- ✅ Use fractional distillation data as key evidence.
- ✅ Apply this understanding to environmental and industrial contexts.
Conclusion: Embracing the Complexity of Air
Air may seem simple, but its classification as a mixture reveals profound insights about the natural world. From the variability of its gases to the ease of industrial separation, every line of evidence points to a physical blend, not a chemical union. This understanding is foundational for fields ranging from meteorology to respiratory medicine. By recognizing air for what it truly is—a dynamic, adaptable mixture—we gain greater appreciation for the delicate balance that sustains life on Earth.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?