Fluorine holds the title of the most reactive nonmetal in the periodic table—and for good reason. Its extreme reactivity shapes everything from industrial chemical processes to biological applications. Unlike most elements that require heat or catalysts to react, fluorine often initiates reactions spontaneously, sometimes explosively. Understanding why fluorine behaves this way requires a deep dive into its atomic architecture, electron configuration, and position within the halogen group.
This article explores the scientific principles behind fluorine’s unrivaled reactivity, explains how its properties compare to other halogens, and highlights both the risks and benefits of harnessing such a powerful element.
Atomic Structure and Electron Configuration
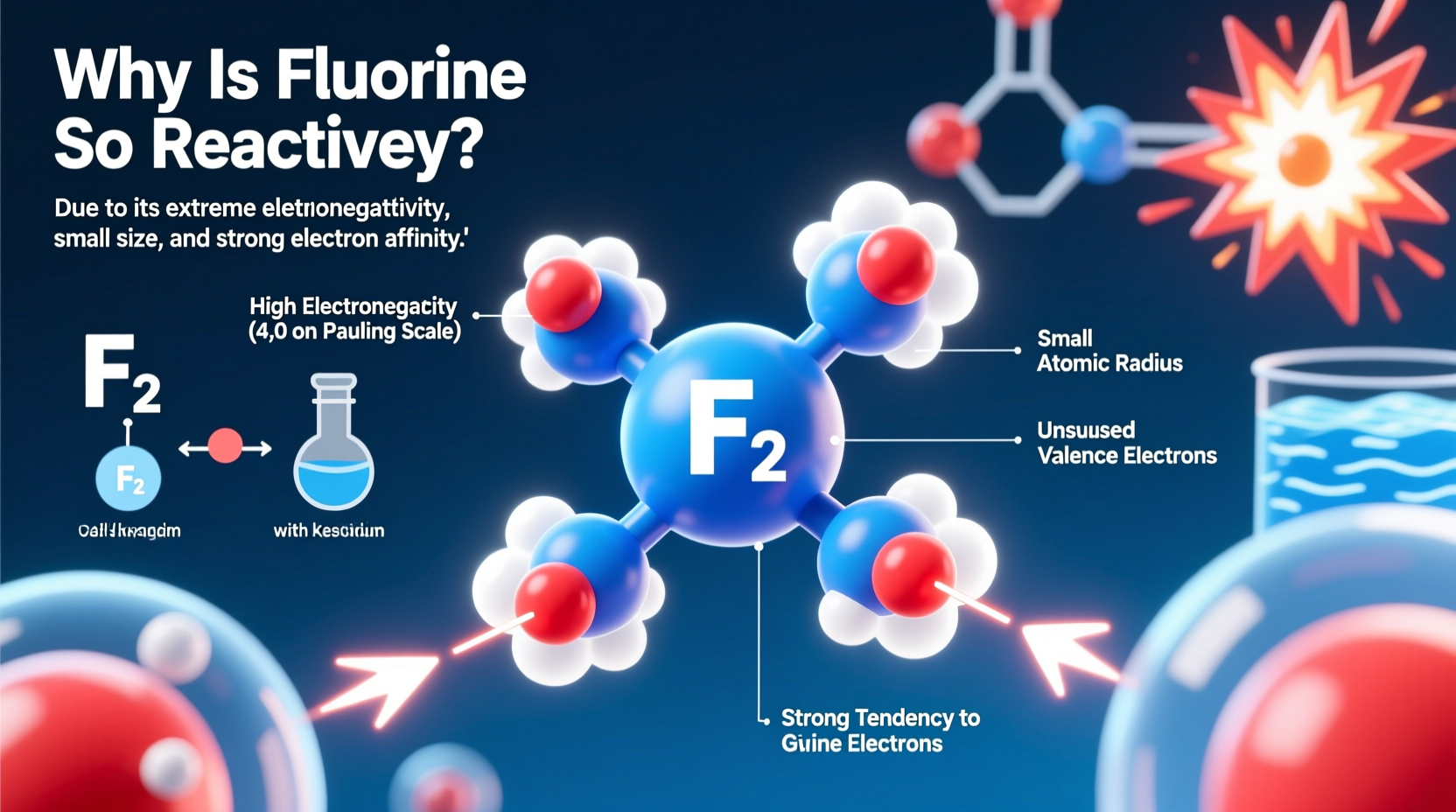
The foundation of fluorine’s reactivity lies in its atomic structure. Fluorine has an atomic number of 9, meaning it contains 9 protons and 9 electrons. Its electron configuration is 1s² 2s² 2p⁵—seven valence electrons in its outer shell. This leaves it just one electron short of achieving a stable octet, the energetically favorable configuration seen in noble gases like neon.
To reach stability, fluorine aggressively seeks to gain that missing electron. This strong desire drives its tendency to form bonds with nearly any available atom, especially those capable of donating electrons. The smaller size of fluorine compared to other halogens amplifies this effect: its nucleus exerts a powerful pull on nearby electrons due to minimal electron shielding and short atomic radius.
Electronegativity: The Driving Force Behind Reactivity
No element pulls electrons more strongly than fluorine. With a Pauling electronegativity value of 3.98—the highest of all elements—it dominates chemical bonding scenarios where electron sharing occurs. This intense attraction makes fluorine exceptionally effective at oxidizing other substances, often stripping electrons away violently.
For example, when fluorine encounters hydrogen, even at low temperatures, they react explosively to form hydrogen fluoride (HF). Similarly, it reacts with metals like sodium not just rapidly, but often with visible flames or detonations. Even normally inert materials like glass, asbestos, and water are not immune; fluorine attacks them vigorously under standard conditions.
“Fluorine doesn’t just participate in reactions—it commands them.” — Dr. Linus Pauling, Nobel Laureate in Chemistry
Comparison with Other Halogens
While all halogens (Group 17) are reactive due to their seven valence electrons, fluorine stands apart. As you move down the group—from fluorine to chlorine, bromine, iodine, and astatine—atomic size increases and electronegativity decreases. Larger atoms have weaker nuclear pull on incoming electrons, making them less eager to acquire an additional one.
Moreover, bond dissociation energy trends reveal another anomaly: despite being smaller, the F–F bond in molecular fluorine (F₂) is surprisingly weak compared to Cl–Cl or Br–Br bonds. This weakness means less energy is required to break F₂ molecules into reactive fluorine atoms, further enhancing its readiness to initiate reactions.
| Halogen | Atomic Radius (pm) | Electronegativity (Pauling) | F–X Bond Strength (kJ/mol) |
|---|---|---|---|
| Fluorine | 72 | 3.98 | 158 |
| Chlorine | 99 | 3.16 | 243 |
| Bromine | 114 | 2.96 | 193 |
| Iodine | 133 | 2.66 | 151 |
The data shows that while fluorine has the smallest atomic radius and highest electronegativity, its diatomic bond strength is lower than chlorine’s—making it easier to activate and therefore more reactive overall.
Safety Challenges and Industrial Applications
Handling fluorine demands extreme caution. Due to its ability to ignite organic matter spontaneously—including skin, wood, and rubber—it must be stored in specialized metal containers (typically nickel or Monel alloy), which form a protective fluoride layer upon exposure. Even trace moisture can lead to hazardous reactions, producing corrosive hydrofluoric acid.
Despite these dangers, fluorine's reactivity is harnessed across industries:
- In uranium enrichment, fluorine is used to produce uranium hexafluoride (UF₆), essential for nuclear fuel processing.
- It serves as a precursor to fluoropolymers like Teflon (PTFE), known for their heat resistance and non-stick properties.
- Pharmaceuticals use fluorinated compounds to enhance drug stability and bioavailability—one-fifth of prescription drugs contain fluorine.
- Fluoride ions derived from fluorine compounds help prevent tooth decay in dental care products.
Mini Case Study: The Manhattan Project and Fluorine Chemistry
During World War II, scientists working on the Manhattan Project faced the challenge of separating fissile uranium-235 from natural uranium. They turned to gaseous diffusion using uranium hexafluoride (UF₆), chosen because fluorine exists as a single isotope (¹⁹F), simplifying mass separation. However, handling UF₆ posed significant risks: it reacts violently with water and corrodes most metals. Engineers developed corrosion-resistant equipment lined with nickel and implemented strict containment protocols. This historic application underscores both the utility and danger of fluorine’s reactivity in large-scale operations.
Step-by-Step Guide: How Fluorine Reacts with Water
One of the most dramatic demonstrations of fluorine’s reactivity is its interaction with water. Here’s what happens step by step:
- Contact: Gaseous fluorine (F₂) comes into contact with liquid water (H₂O).
- Bond Breaking: Fluorine molecules dissociate easily due to weak F–F bonds, forming highly reactive fluorine atoms.
- Oxidation: Fluorine oxidizes water, stealing electrons and breaking H–O bonds.
- Products Formed: The primary reaction produces hydrofluoric acid (HF) and oxygen gas (O₂), though some ozone (O₃) may also form.
- Energy Release: Significant heat is released, often igniting the hydrogen gas produced in side reactions.
The net reaction can be summarized as:
2F₂ + 2H₂O → 4HF + O₂
This process illustrates how fluorine doesn’t merely dissolve in water—it destroys it through oxidation.
FAQ
Can fluorine react with noble gases?
Yes, under specific laboratory conditions, fluorine can react with certain noble gases. For instance, xenon combines with fluorine to form xenon difluoride (XeF₂), xenon tetrafluoride (XeF₄), and others. These were among the first noble gas compounds ever synthesized, challenging the long-held belief that noble gases were entirely inert.
Why isn’t fluorine used more widely if it’s so reactive?
Its extreme reactivity makes fluorine difficult and dangerous to handle. Most industrial processes use safer fluorine-containing compounds (like HF or SF₆) instead of elemental fluorine. Additionally, storage, transport, and reaction control require specialized infrastructure, limiting its widespread direct use.
Is fluorine essential for life?
Elemental fluorine is too toxic for biological systems, but fluoride ions (F⁻) play a beneficial role in strengthening tooth enamel and preventing cavities. However, excessive intake can lead to fluorosis, so balance is crucial.
Checklist: Safe Handling Practices for Fluorine Compounds
- ✅ Use only chemically resistant gloves and face shields when handling fluorinated agents.
- ✅ Store fluoride solutions in plastic containers—not glass—to avoid etching.
- ✅ Ensure proper ventilation in labs using volatile fluorine compounds.
- ✅ Neutralize spills with calcium-based absorbents to precipitate non-toxic CaF₂.
- ✅ Never mix fluorine compounds with organic solvents without rigorous risk assessment.
Conclusion
Fluorine’s unmatched reactivity stems from a perfect storm of atomic traits: high electronegativity, small size, low bond dissociation energy, and a relentless drive to complete its valence shell. These characteristics make it both a marvel of chemistry and a formidable hazard. From protecting teeth to powering nuclear reactors, fluorine’s influence spans science and industry—but only when handled with respect and precision.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?