Lomotil, a prescription medication used to treat acute diarrhea, contains two active ingredients: diphenoxylate and atropine. While effective in managing severe gastrointestinal symptoms, it’s classified as a controlled substance due to its potential for misuse and dependence. This classification raises important questions about its safety, regulation, and appropriate use. Understanding the reasons behind its controlled status, along with its medical applications and legal framework, is essential for patients and caregivers alike.
What Is Lomotil and How Does It Work?
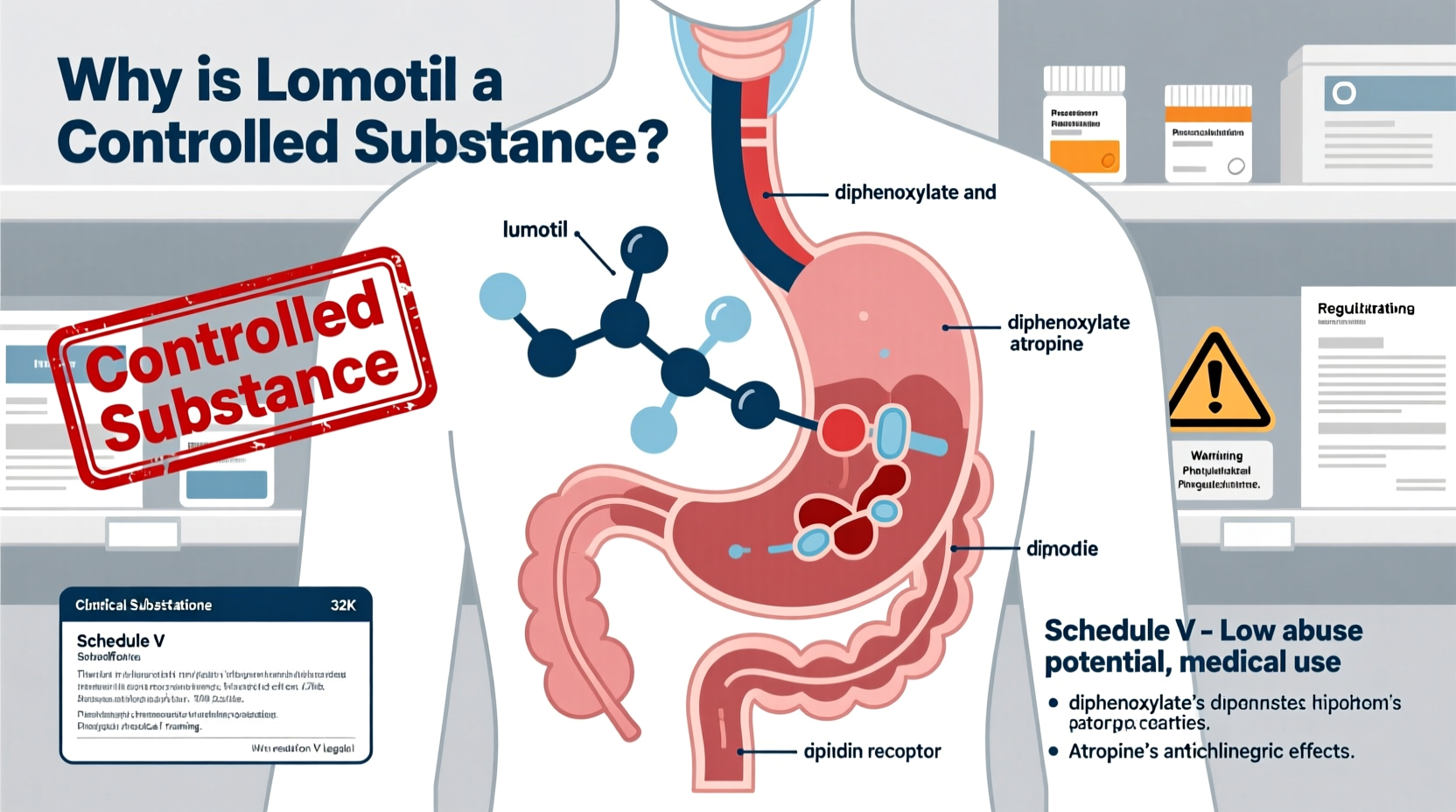
Lomotil (generic name: diphenoxylate/atropine) is an anti-diarrheal medication typically prescribed when other treatments fail or when rapid control of bowel movements is needed. Diphenoxylate, the primary active ingredient, is chemically related to opioids and acts on opioid receptors in the intestines to slow down gut motility. This reduces the frequency of bowel movements by allowing more time for fluid absorption in the colon.
The addition of atropine—a mild anticholinergic agent—serves as a deterrent to abuse. At therapeutic doses, atropine has minimal effects, but if someone attempts to take large quantities of Lomotil to achieve euphoria, the atropine causes unpleasant side effects such as dry mouth, blurred vision, tachycardia, and urinary retention.
“Diphenoxylate has central nervous system activity similar to opioids, which necessitates careful prescribing and monitoring.” — Dr. Rachel Nguyen, Gastroenterology Specialist
Why Is Lomotil Classified as a Controlled Substance?
In the United States, Lomotil is classified as a Schedule V controlled substance under the Controlled Substances Act (CSA). This means it has a lower potential for abuse compared to drugs like heroin or cocaine (Schedule I–II), but still carries risks significant enough to warrant regulatory oversight.
The key reasons for this classification include:
- Opioid-like properties: Diphenoxylate is structurally similar to meperidine (Demerol), a known opioid analgesic. Though it primarily acts peripherally in the gut, it can cross the blood-brain barrier in high doses, leading to central effects including euphoria.
- Potential for dependence: Long-term or excessive use may lead to physical dependence and withdrawal symptoms upon discontinuation, similar to other opioids.
- History of misuse: There have been documented cases of individuals crushing and injecting Lomotil to bypass the atropine deterrent, seeking opioid-like highs.
- Overdose risk: In children or sensitive individuals, even standard doses can cause respiratory depression, especially if combined with other CNS depressants.
Medical Uses and Approved Indications
Lomotil is not intended for routine or chronic diarrhea management. It is generally reserved for short-term treatment of acute diarrhea when non-pharmacological interventions (like hydration and dietary adjustments) are insufficient.
Common clinical scenarios where Lomotil may be prescribed include:
- Severe traveler’s diarrhea unresponsive to rehydration
- Diarrhea associated with inflammatory bowel disease flare-ups (used cautiously)
- Preparation for certain diagnostic procedures requiring bowel quieting
- Palliative care settings to manage intractable diarrhea
It is contraindicated in patients with pseudomembranous colitis (e.g., C. difficile infection), obstructive bowel disorders, or known hypersensitivity to its components. Use in children under 2 years old is strongly discouraged due to increased risk of respiratory depression.
Regulatory Framework and Prescription Guidelines
As a Schedule V drug, Lomotil is subject to specific federal and state regulations designed to prevent diversion and abuse while ensuring legitimate medical access.
Key regulatory aspects include:
- Prescription requirement: Lomotil cannot be purchased over-the-counter; a valid prescription from a licensed healthcare provider is required.
- No refills without new prescription: Unlike some lower-schedule medications, Schedule V drugs like Lomotil allow limited refills (up to five within six months), but many pharmacies and insurers require a new script each time.
- Pharmacy monitoring: Sales are tracked through state Prescription Drug Monitoring Programs (PDMPs), which flag unusual prescribing patterns.
- Storage and disposal: Patients are advised to store Lomotil securely and dispose of unused portions properly to prevent accidental ingestion or misuse by others.
| Controlled Substance Schedule | Abuse Potential | Lomotil Classification | Typical Medical Use |
|---|---|---|---|
| Schedule I | High – No accepted medical use | ❌ Not applicable | None (e.g., heroin, LSD) |
| Schedule II | High – Severe dependence risk | ❌ | Strong painkillers (e.g., oxycodone) |
| Schedule V | Low – Limited dependence risk | ✅ Yes | Antidiarrheals with small opioid amounts |
Safe Use Practices and Risk Mitigation
To minimize risks associated with Lomotil, both prescribers and patients must follow evidence-based guidelines for safe use.
Step-by-Step Guide to Responsible Lomotil Use
- Confirm diagnosis: Rule out infectious causes (e.g., bacterial gastroenteritis) before starting Lomotil, as slowing gut motility can prolong infection.
- Start low, go slow: Begin with the lowest effective dose—typically one tablet three to four times daily.
- Limit duration: Use for no more than 48 hours unless directed otherwise by a physician.
- Monitor for side effects: Watch for drowsiness, constipation, abdominal distension, or signs of CNS depression.
- Avoid alcohol and sedatives: These can amplify respiratory depression and sedation.
- Discontinue gradually: After prolonged use, taper off slowly to prevent withdrawal symptoms like anxiety, sweating, and insomnia.
Real-World Scenario: When Lomotil Use Goes Wrong
A 34-year-old man with a history of opioid use disorder was prescribed Lomotil for persistent diarrhea following food poisoning. Without disclosing his substance use history, he began taking double the recommended dose, reporting that it “calmed his nerves.” Within a week, he experienced dizziness, confusion, and shallow breathing. He was hospitalized after a family member found him disoriented. Toxicology screening confirmed diphenoxylate toxicity. This case highlights the importance of patient disclosure, cautious prescribing in high-risk individuals, and the real dangers of exceeding therapeutic doses—even with lower-potency opioids.
Frequently Asked Questions
Can Lomotil get you high?
At normal doses, Lomotil does not produce euphoria due to the presence of atropine and the low concentration of diphenoxylate. However, taking significantly higher doses than prescribed may result in mild opioid-like effects, though this also triggers uncomfortable anticholinergic symptoms and poses serious health risks.
Is Lomotil addictive?
Yes, especially with prolonged or excessive use. Physical dependence can develop, leading to withdrawal symptoms such as restlessness, nausea, sweating, and tremors upon abrupt discontinuation. It should only be used under medical supervision and for short durations.
How does Lomotil differ from Imodium (loperamide)?
Both are anti-diarrheal agents that act on intestinal opioid receptors. However, loperamide does not cross the blood-brain barrier significantly and is not federally scheduled (though some states regulate high-dose forms). Lomotil, containing diphenoxylate, has greater CNS penetration and is therefore classified as a controlled substance.
Conclusion: Balancing Efficacy and Safety
Lomotil remains a valuable tool in managing acute diarrhea when used appropriately. Its classification as a Schedule V controlled substance reflects a necessary balance between therapeutic benefit and public health protection. By understanding its mechanism, risks, and regulatory context, patients and providers can make informed decisions that prioritize safety without denying access to effective treatment.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?