The ocean covers more than 70% of Earth’s surface, yet one of its most basic characteristics often goes unexamined: why is it salty? It’s easy to take for granted that seawater tastes briny, but the answer lies in a complex, ongoing natural process involving geology, chemistry, and climate. Understanding ocean salinity isn’t just fascinating—it helps explain everything from weather patterns to marine ecosystems. This article breaks down the science behind saltwater in clear, straightforward terms, revealing how rain, rocks, rivers, and even underwater volcanoes contribute to the sea’s signature taste.
What Makes Seawater Salty?
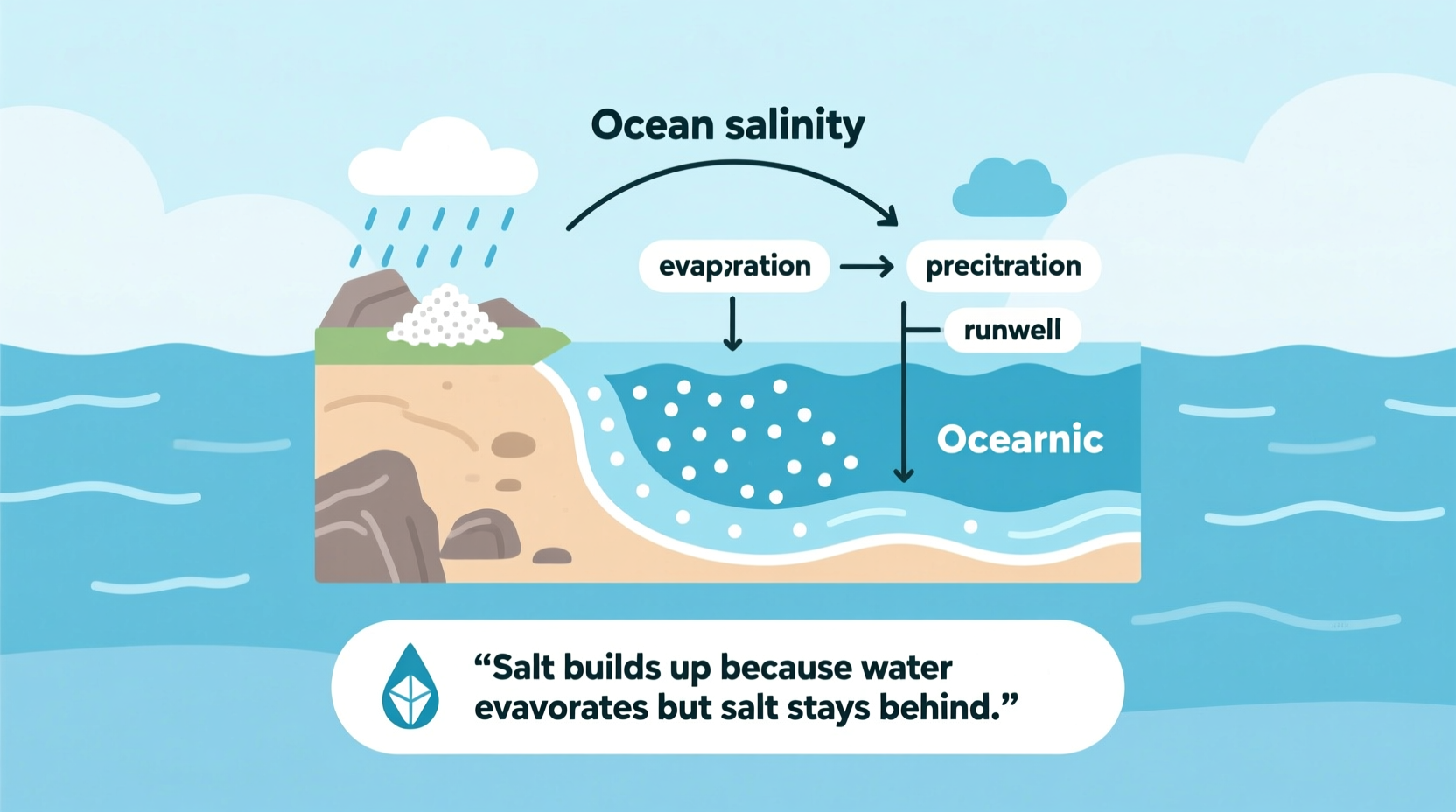
Ocean water contains dissolved salts—mostly sodium chloride, the same compound found in table salt. On average, every liter of seawater holds about 35 grams of dissolved solids, a measurement known as salinity (typically expressed as 35 parts per thousand or 3.5%). But these salts didn’t originate in the ocean. Instead, they were delivered over millions of years through a continuous cycle of erosion, runoff, and chemical exchange.
Rainwater is naturally slightly acidic due to dissolved carbon dioxide from the atmosphere. As it falls to Earth, it interacts with rocks and soil, slowly dissolving minerals like sodium, calcium, magnesium, and potassium. These ions are carried by rivers into lakes and eventually into the ocean. Once there, they accumulate because the ocean has no natural “drain.” Unlike freshwater systems, where water continually cycles out through evaporation and precipitation, the ocean acts as a long-term reservoir for dissolved minerals.
The Role of Volcanic Activity and Hydrothermal Vents
While river runoff contributes the majority of dissolved ions, another significant source of ocean salinity comes from beneath the seafloor. Underwater volcanic activity and hydrothermal vents release minerals directly into seawater. When seawater seeps into cracks in the ocean crust, it's heated by magma, triggering chemical reactions that leach elements like chlorine, sulfur, and iron from surrounding rocks. This superheated, mineral-rich water then erupts back into the ocean through vents, adding to the overall salt content.
This deep-sea contribution may seem minor compared to surface runoff, but over geological timescales, it plays a crucial role. Scientists estimate that hydrothermal systems recycle a substantial portion of oceanic sodium and chloride, helping regulate long-term salinity levels.
“Hydrothermal circulation is like Earth’s internal filtration system—it doesn’t just add salt; it transforms and redistributes it.” — Dr. Lena Torres, Marine Geochemist, Scripps Institution of Oceanography
Why Doesn’t the Ocean Keep Getting Saltier Forever?
If rivers continuously deliver salt and underwater sources add more, why hasn’t the ocean become a solid block of salt over billions of years? The answer lies in natural removal processes that balance the input.
Several mechanisms prevent runaway salinization:
- Sedimentation: Some salts bind to particles and sink to the seafloor, becoming part of sedimentary rock over time.
- Sea spray: Wind and waves eject tiny droplets into the air; when they evaporate, salt particles fall back to land, removing small amounts from the ocean.
- Biological uptake: Marine organisms like corals and shellfish use calcium and carbonate to build skeletons, effectively locking away certain ions.
- Evaporation and precipitation cycles: In regions with high evaporation (like the Red Sea), salinity temporarily increases, but rainfall and river inflow dilute other areas, maintaining global equilibrium.
This dynamic balance means that while local salinity varies, the overall concentration of salt in the world’s oceans has remained relatively stable for hundreds of thousands of years.
Regional Differences in Salinity
Not all parts of the ocean are equally salty. Salinity fluctuates based on location, climate, and geography. For example, the Dead Sea is so saturated with salt that nothing can live in it, while polar seas near melting glaciers are much fresher.
| Region | Average Salinity (ppt) | Key Influencing Factors |
|---|---|---|
| Open Ocean (Global Average) | 35 | Balanced evaporation and precipitation |
| Red Sea | 41 | High evaporation, low rainfall, limited freshwater input |
| Baltic Sea | 10–15 | Heavy river inflow, cold climate, limited connection to open ocean |
| Amazon River Mouth | 10–20 | Freshwater discharge dilutes seawater |
| Antarctic Waters | 33–34 | Ice melt reduces salinity; dense cold water sinks despite lower salt |
These variations affect ocean density, which in turn drives deep-water currents and influences global climate. High-salinity water is denser and tends to sink, forming deep ocean currents that transport heat around the planet—a key component of Earth’s climate regulation system.
How Salinity Affects Marine Life
Most marine species have evolved to thrive within a narrow range of salinity. Sudden changes—such as those caused by storm runoff or glacial melt—can stress or kill aquatic organisms. Fish, for instance, must constantly regulate the salt concentration in their bodies through a process called osmoregulation. They drink seawater and excrete excess salt through their gills, requiring significant energy.
In estuaries, where rivers meet the sea, salinity shifts daily with tides. Species like oysters, crabs, and certain fish have adapted to tolerate these fluctuations, making estuaries rich but fragile ecosystems. However, human activities such as dam construction and excessive freshwater diversion can disrupt this balance, leading to habitat degradation.
Mini Case Study: The Aral Sea Crisis
The Aral Sea, once one of the largest lakes in the world, provides a stark example of how altering water flow impacts salinity and ecology. Beginning in the 1960s, Soviet irrigation projects diverted rivers that fed the lake. With less freshwater inflow, evaporation concentrated the remaining water, causing salinity to skyrocket—from about 10 ppt to over 100 ppt. The increased salt levels killed nearly all native fish species, collapsed local fisheries, and turned once-thriving coastal communities into ghost towns. This man-made disaster underscores how delicate the balance of salinity can be—even in inland bodies of water.
FAQ: Common Questions About Ocean Salinity
Can you drink ocean water if you boil it?
No. Boiling seawater kills microbes but does not remove salt. To make it safe to drink, desalination is required—either through distillation (boiling and condensing steam) or reverse osmosis (forcing water through a membrane that filters out salts).
Is the ocean getting saltier due to climate change?
Some regions are experiencing higher salinity due to increased evaporation from warming temperatures, while others are becoming fresher from accelerated ice melt. Overall, the total salt content isn’t rising dramatically, but the distribution is shifting, which can affect ocean circulation and weather patterns.
Are there any completely salt-free oceans?
No. All oceans contain dissolved salts. However, marginal seas and coastal areas with heavy freshwater input—like the Gulf of Finland or the mouth of the Amazon—can have very low salinity, approaching that of brackish water.
Step-by-Step: How Rainwater Becomes Seawater
- Rain falls on land – Slightly acidic due to atmospheric CO₂, it begins dissolving minerals in rocks.
- Erosion transports ions – Rivers carry dissolved sodium, chloride, calcium, and other elements toward the sea.
- Runoff enters the ocean – Salts accumulate because there’s no natural outlet for them to escape.
- Evaporation removes pure water – Sunlight turns liquid water into vapor, leaving salts behind and increasing concentration.
- Cycles continue – Over time, equilibrium is maintained through sedimentation, biological uptake, and deep-ocean processes.
Checklist: Key Facts About Ocean Salinity
- ✅ Most ocean salt comes from eroded rocks carried by rivers.
- ✅ Sodium and chloride make up about 85% of dissolved salts.
- ✅ Hydrothermal vents contribute minerals from beneath the seafloor.
- ✅ Salinity averages 35 parts per thousand globally.
- ✅ Natural removal processes keep salt levels balanced over time.
- ✅ Changes in salinity affect ocean currents and marine life.
Conclusion
The saltiness of the ocean is not a static condition but the result of a dynamic, planet-scale process that has unfolded over eons. From the slow weathering of mountains to the hidden churning of undersea vents, Earth’s geology and climate work together to maintain the delicate balance of seawater. Understanding salinity helps us appreciate not only the complexity of the oceans but also their vulnerability to human influence. As climate change alters precipitation and ice melt patterns, monitoring salinity becomes increasingly important for predicting environmental shifts.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?