Water is often referred to as the \"universal solvent\" — a title that reflects its unparalleled ability to dissolve more substances than any other liquid on Earth. This property isn't just a scientific curiosity; it's foundational to biological processes, environmental systems, and industrial applications. From transporting nutrients in your bloodstream to weathering rocks over millennia, water’s dissolving power shapes life and landscapes alike. But what exactly gives water this remarkable capability? The answer lies in its molecular structure, polarity, and hydrogen bonding — features that allow it to interact with an extraordinary range of compounds.
The Chemistry Behind Water’s Solvent Power
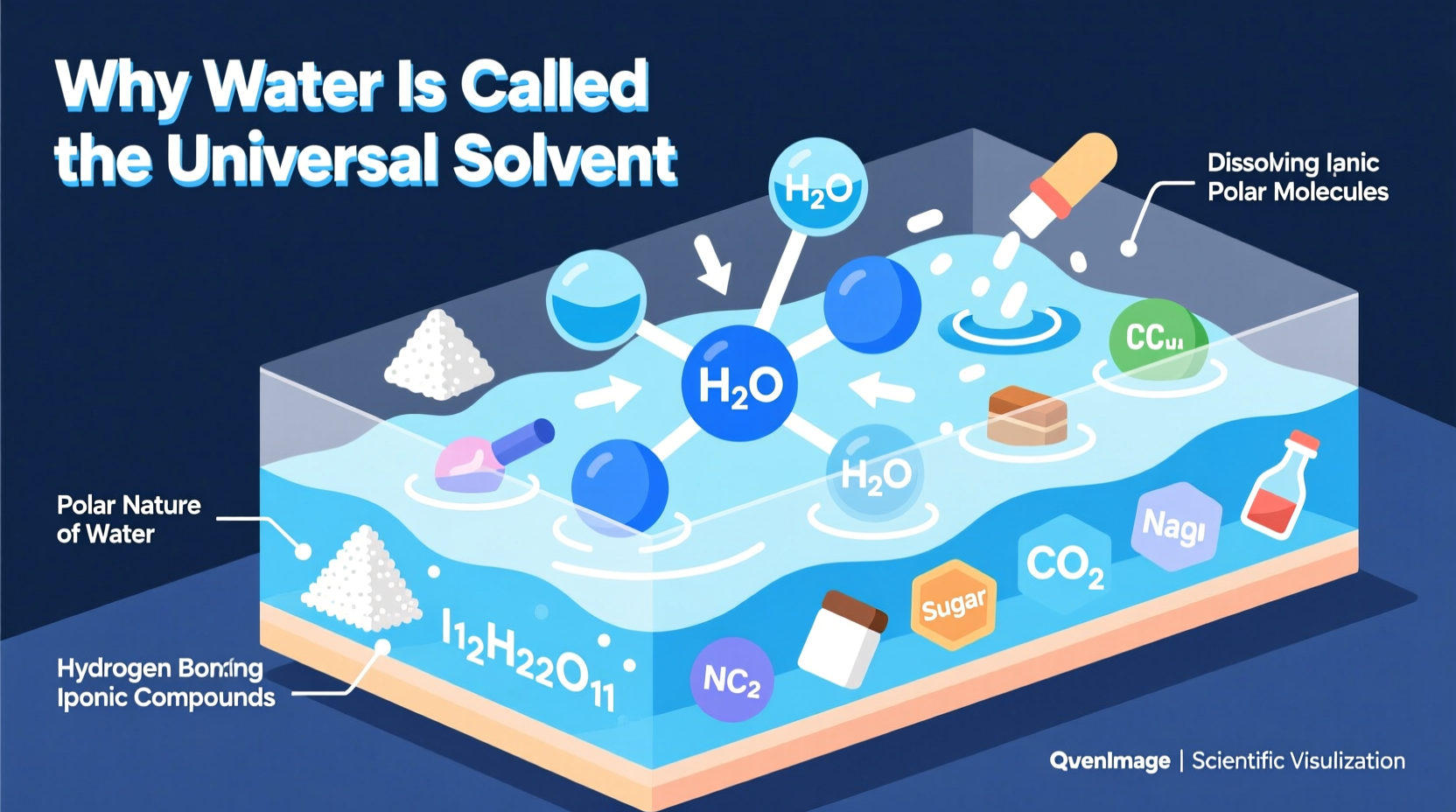
At the heart of water’s effectiveness as a solvent is its polar molecular structure. A water molecule (H₂O) consists of two hydrogen atoms bonded to one oxygen atom in a bent shape, creating an uneven distribution of electrical charge. Oxygen is more electronegative than hydrogen, meaning it pulls electrons closer to itself. This results in a partial negative charge (δ⁻) on the oxygen end and partial positive charges (δ⁺) on the hydrogen ends.
This polarity enables water molecules to surround and interact with charged or polar substances. When ionic compounds like table salt (NaCl) enter water, the positively charged sodium ions (Na⁺) are attracted to the oxygen side of water molecules, while the negatively charged chloride ions (Cl⁻) are drawn to the hydrogen sides. This process, called dissociation, pulls the ions apart and disperses them evenly throughout the solution — effectively dissolving the compound.
Hydrogen Bonding: The Glue That Makes It Work
Beyond simple polarity, water’s ability to form hydrogen bonds significantly enhances its solvent properties. Each water molecule can form up to four hydrogen bonds with neighboring molecules, creating a dynamic, interconnected network. These bonds are strong enough to stabilize dissolved particles but weak enough to allow constant movement and interaction.
Hydrogen bonding also contributes to water’s high surface tension, specific heat capacity, and boiling point — all of which support its role in sustaining life. In solution, these bonds help shield dissolved ions from recombining, keeping them suspended and available for biological or chemical reactions.
“Water’s combination of polarity and hydrogen bonding creates a uniquely versatile medium for biochemical reactions.” — Dr. Linda Chen, Biochemist at MIT
Why “Universal” Doesn’t Mean “Everything Dissolves”
While water dissolves many substances, it does not dissolve all. Nonpolar compounds such as oils, fats, and waxes resist dissolution in water due to their lack of charge separation. These hydrophobic (\"water-fearing\") substances tend to cluster together when mixed with water, minimizing contact with polar water molecules.
This limitation underscores an important nuance: calling water the \"universal solvent\" refers to its broad compatibility with ionic and polar substances — not universal solubility across all matter. However, given that most biologically and geologically relevant chemicals fall into the polar or ionic category, water’s reach remains exceptionally wide.
Biological Importance of Water as a Solvent
In living organisms, water’s solvent properties are indispensable. Inside cells, water dissolves ions, sugars, amino acids, and other vital molecules, enabling metabolic pathways to proceed efficiently. Blood plasma, largely composed of water, transports dissolved nutrients, hormones, and waste products throughout the body. Even digestion relies on aqueous solutions — saliva, gastric juices, and bile all use water as a base to break down food components.
Photosynthesis, one of the most critical processes on Earth, depends on water not only as a reactant but also as the medium in which chloroplasts manage ion gradients and enzyme activity. Without water’s ability to dissolve and mobilize key reactants, energy conversion in plants would be impossible.
Environmental and Industrial Roles
Nature uses water’s solvent power extensively. Rainwater absorbs carbon dioxide from the atmosphere, forming weak carbonic acid (H₂CO₃), which slowly dissolves limestone and contributes to cave formation. This process, known as chemical weathering, reshapes landscapes over time and releases essential minerals into ecosystems.
In agriculture, soil moisture carries dissolved nutrients like nitrates, phosphates, and potassium ions to plant roots. Farmers rely on irrigation not just to hydrate crops but to ensure nutrient availability through aqueous transport.
Industrially, water serves as a primary solvent in manufacturing, pharmaceuticals, and cleaning agents. Its low toxicity, abundance, and effectiveness make it preferable to organic solvents whenever possible. Wastewater treatment facilities exploit water’s solvency to separate contaminants through precipitation, filtration, and biological processing.
Step-by-Step: How Water Dissolves Salt
- A grain of salt (NaCl) is introduced into water.
- Polar water molecules orient themselves around the crystal: oxygen ends face Na⁺ ions; hydrogen ends face Cl⁻ ions.
- Electrostatic attraction between water dipoles and ions weakens the ionic bonds holding the crystal together.
- Ions detach from the crystal lattice and become surrounded by hydration shells of water molecules.
- Dissolved ions disperse uniformly throughout the solution, resulting in a homogeneous mixture.
Do’s and Don’ts of Using Water as a Solvent
| Do’s | Don’ts |
|---|---|
| Use distilled water for sensitive lab experiments to avoid interference from dissolved minerals. | Assume tap water is pure — it contains chlorine, calcium, and other dissolved substances. |
| Leverage water’s polarity to extract polar compounds in herbal infusions or tinctures. | Use water to clean electronic components — conductivity from dissolved ions can cause short circuits. |
| Store aqueous solutions in sealed containers to prevent evaporation and contamination. | Mix water with nonpolar solvents expecting uniform mixing — they will separate into layers. |
Frequently Asked Questions
Can water dissolve metals?
Pure water doesn’t dissolve solid metals, but it can facilitate corrosion. For example, iron reacts with water and oxygen to form rust (iron oxide), which can flake away, giving the appearance of dissolution. Some alkali metals like sodium react violently with water, producing soluble hydroxides.
Is seawater more effective at dissolving substances than freshwater?
No — seawater already contains high concentrations of dissolved salts, reducing its capacity to dissolve additional ionic compounds. Freshwater generally has greater solvent potential due to lower initial solute content.
Does temperature affect water’s solvent ability?
Yes. Higher temperatures increase molecular motion, allowing water to break apart solute particles more quickly. Most solids dissolve faster in hot water, though gases like oxygen become less soluble as temperature rises.
Real-World Example: Kidney Function and Water’s Role
The human kidney provides a compelling illustration of water’s solvent function. Every day, kidneys filter about 180 liters of blood plasma, removing waste products like urea, excess ions, and toxins. These substances must be sufficiently soluble in water to pass through filtration membranes and be excreted in urine. Patients with kidney stones often suffer because certain minerals (like calcium oxalate) exceed their solubility limit in urine, crystallizing into painful deposits. Doctors frequently recommend increased water intake to dilute urine and prevent stone formation — a direct application of water’s solvent principle in preventive medicine.
Conclusion: Embracing Water’s Unique Nature
Water earns its title as the universal solvent not through exaggeration, but through exceptional molecular design. Its polarity, hydrogen bonding, and abundance enable it to mediate countless chemical interactions essential to life, industry, and Earth’s natural systems. Understanding why water dissolves what it does — and why some things remain insoluble — empowers better decisions in health, science, and environmental stewardship.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?