Phosphorus is one of the essential building blocks of life. Unlike carbon or nitrogen, it does not have a gaseous phase, meaning it cycles slowly through rocks, water, soil, and living organisms. This makes the phosphorus cycle unique—and critically important. From forming DNA to fueling plant growth, phosphorus plays a non-negotiable role in sustaining ecosystems and human agriculture. Yet, disruptions to this cycle are contributing to environmental degradation worldwide. Understanding how phosphorus moves through the environment, why it's indispensable, and how we can manage it responsibly is vital for long-term ecological and food security.
The Role of Phosphorus in Living Systems
Phosphorus is a key component of nucleic acids like DNA and RNA, which carry genetic information in all living organisms. It also forms part of adenosine triphosphate (ATP), the molecule that stores and transfers energy within cells. Without phosphorus, cellular respiration, photosynthesis, and reproduction would not be possible. In plants, phosphorus supports root development, flowering, and seed production. In animals, it contributes to bone and teeth formation and helps regulate metabolic processes.
Because organisms cannot synthesize phosphorus, they must obtain it from their environment—primarily through food or absorption from soil and water. This dependency makes the availability of phosphorus a limiting factor in many ecosystems. When phosphorus is scarce, biological productivity slows down. Conversely, excess phosphorus can lead to harmful overgrowth of algae and aquatic plants, disrupting entire aquatic systems.
How the Phosphorus Cycle Works
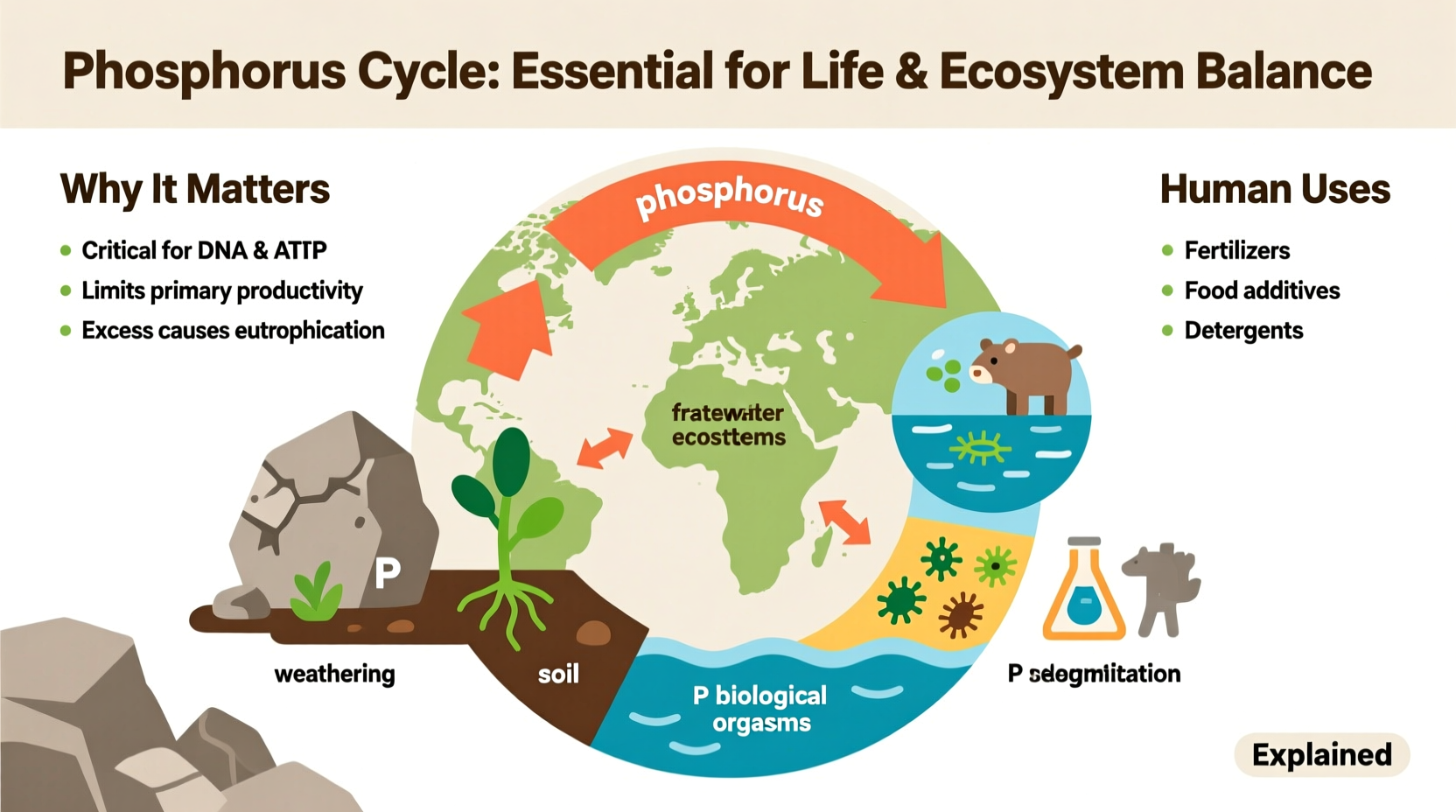
The phosphorus cycle is sedimentary, meaning it primarily moves through land, water, and sediments rather than the atmosphere. The cycle begins with the slow weathering of phosphate-containing rocks. Over thousands of years, rain and physical erosion release inorganic phosphate ions into soil and waterways. Plants absorb these phosphates through their roots, incorporating them into organic compounds. Herbivores then consume the plants, and carnivores eat the herbivores, transferring phosphorus up the food chain.
When organisms die, decomposers break down organic matter, returning phosphorus to the soil as inorganic phosphate. Some of this phosphate leaches into rivers and lakes, eventually settling into ocean sediments. Over geological timescales, tectonic activity may uplift these sediments, forming new phosphate-rich rock and restarting the cycle.
“Phosphorus is the ultimate finite resource—there’s no substitute for it in biology, and once lost to oceans, it’s effectively gone for millennia.” — Dr. Dana Cordell, Researcher in Sustainable Phosphorus Management
Agricultural Dependence and Fertilizer Use
Modern agriculture relies heavily on phosphorus fertilizers to maintain high crop yields. Since natural soil phosphorus levels are often insufficient for intensive farming, synthetic fertilizers derived from mined phosphate rock are applied to fields globally. While this has dramatically increased food production since the 20th century, it has also accelerated the phosphorus cycle beyond natural rates.
About 22 million metric tons of phosphorus are mined annually, mostly for fertilizer. However, only a fraction reaches crops; much of it runs off into water bodies during rainfall, leading to eutrophication—a process where excess nutrients cause algal blooms that deplete oxygen and kill aquatic life.
| Stage | Natural Process | Human Impact |
|---|---|---|
| Weathering | Slow release from rocks | Accelerated by mining |
| Uptake | Plants absorb phosphate | Fertilizers boost uptake |
| Runoff | Minimal loss to water | High runoff from farms |
| Sedimentation | Gradual ocean deposition | Rapid accumulation in lakes |
| Recycling | Via decomposition | Limited due to waste disposal |
Environmental Consequences of Imbalance
Disruptions in the phosphorus cycle have led to significant environmental issues. One of the most visible is freshwater eutrophication. Lakes such as Lake Erie in North America and Taihu in China have experienced recurring toxic algal blooms due to phosphorus runoff from agricultural lands and wastewater discharge. These events harm biodiversity, contaminate drinking water, and incur massive cleanup costs.
Ocean dead zones—areas with little to no oxygen—are another consequence. Excess phosphorus and nitrogen stimulate plankton growth; when these organisms die and decompose, oxygen is consumed faster than it can be replenished. The Gulf of Mexico hosts one of the largest dead zones, spanning thousands of square miles at its peak.
Moreover, phosphorus is a non-renewable resource. Current estimates suggest economically viable phosphate rock reserves could be depleted within 50 to 100 years if consumption continues unchecked. Unlike nitrogen, which can be fixed from the atmosphere, there is no synthetic replacement for phosphorus in biological systems.
Mini Case Study: Restoring Lake Winnipeg
Lake Winnipeg in Canada faced severe eutrophication due to phosphorus inflow from agricultural runoff and municipal wastewater. By the early 2000s, algal blooms covered large sections of the lake, threatening fisheries and tourism. In response, Manitoba implemented a watershed management strategy focusing on buffer strips, wetland restoration, and upgraded wastewater treatment plants. Over a decade, total phosphorus loading decreased by nearly 50%. While challenges remain, the case demonstrates that targeted interventions can reverse phosphorus pollution and restore ecosystem health.
Strategies for Sustainable Phosphorus Management
To protect both food systems and the environment, sustainable phosphorus stewardship is essential. This involves reducing waste, improving efficiency, and recycling phosphorus wherever possible. Below is a practical checklist for individuals, farmers, and policymakers:
Sustainable Phosphorus Checklist
- ✅ Apply fertilizers based on soil testing—not guesswork
- ✅ Use precision agriculture techniques to minimize over-application
- ✅ Promote the use of cover crops to reduce runoff
- ✅ Upgrade wastewater treatment to recover phosphorus from sewage
- ✅ Support research into struvite recovery and bio-based fertilizers
- ✅ Encourage dietary shifts to reduce demand for phosphorus-intensive animal feed
Innovations such as urine-diverting toilets and phosphorus-recovery systems in treatment plants are already being piloted in Sweden and the Netherlands. These technologies extract phosphorus in the form of struvite, a slow-release fertilizer, turning waste into a valuable resource.
Frequently Asked Questions
Why doesn’t phosphorus cycle through the atmosphere like carbon or nitrogen?
Phosphorus lacks a gaseous state under normal Earth conditions. It primarily exists in solid form within rocks and minerals, so its movement depends on physical erosion, water flow, and biological uptake—not atmospheric circulation.
Can we recycle phosphorus effectively?
Yes, but current recycling rates are low. Human and animal waste contain significant amounts of phosphorus. With proper infrastructure, up to 80% of excreted phosphorus could be recovered and reused in agriculture, reducing reliance on mining.
Is organic farming better for the phosphorus cycle?
Organic farming avoids synthetic fertilizers but still requires phosphorus inputs, often from rock phosphate or manure. While it typically promotes soil health and reduces runoff, sustainability depends on sourcing and managing these inputs responsibly.
Conclusion: A Call for Responsible Stewardship
The phosphorus cycle is silent but foundational—a hidden engine driving life on Earth. Its disruption threatens both natural ecosystems and global food production. Yet, with informed choices, technological innovation, and policy action, we can rebalance this cycle. Farmers can optimize fertilizer use, cities can reclaim phosphorus from waste, and consumers can support sustainable agriculture. Every effort counts in preserving this irreplaceable element for future generations.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?