Everyday experiences—from dropping a rock in water to watching an iceberg drift—raise a simple but profound question: Why do some objects float while others sink? The answer lies in two fundamental scientific concepts: density and buoyancy. These principles govern not only what happens when you toss a coin into a fountain, but also how massive ships stay afloat and submarines dive and resurface. Understanding them reveals the invisible forces shaping our physical world.
The Role of Density in Floating and Sinking
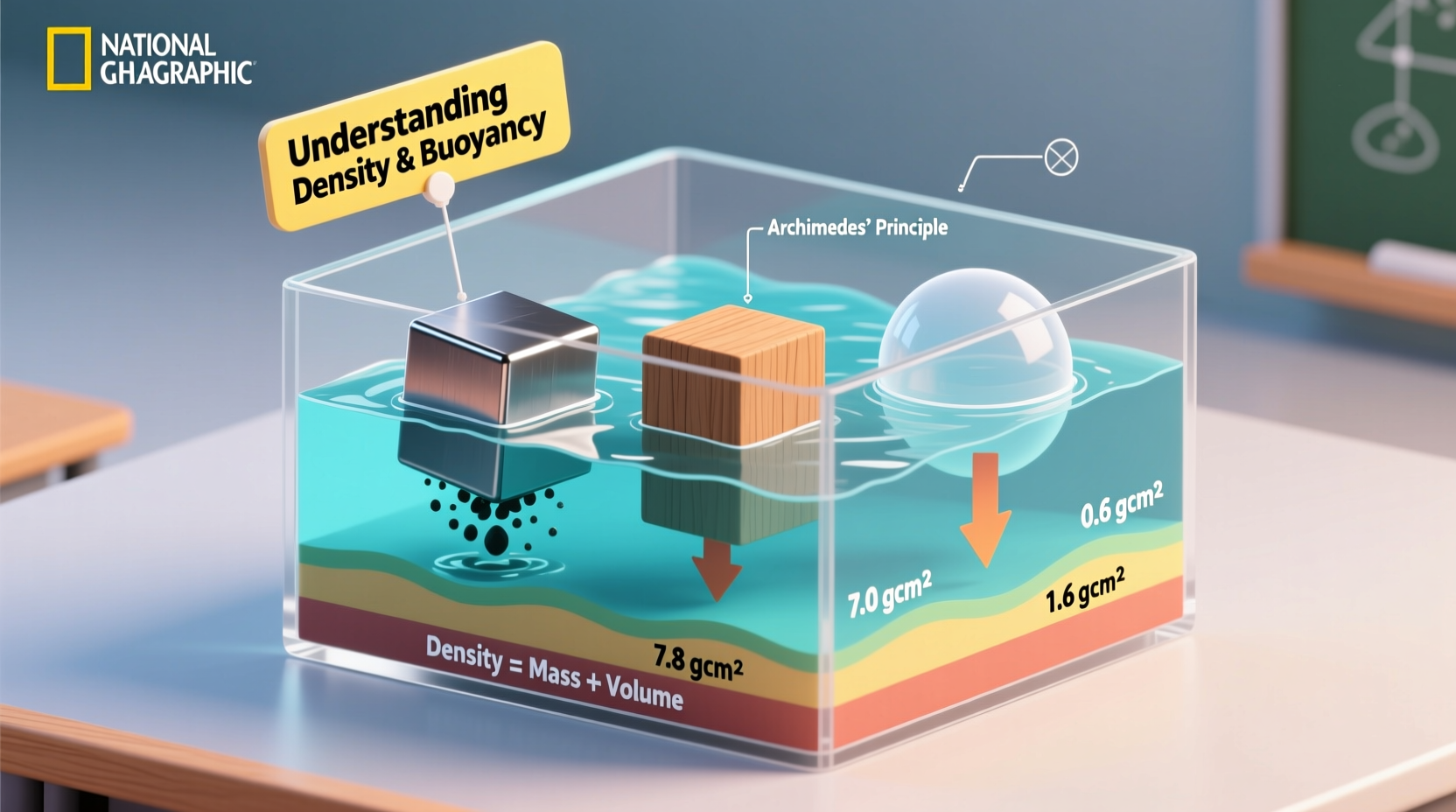
Density is a measure of how much mass is packed into a given volume. It’s calculated using the formula: density = mass / volume. When comparing an object to the fluid it’s placed in—usually water—its relative density determines whether it floats or sinks.
If an object is less dense than the fluid, it will float. If it’s more dense, it will sink. For example, a block of wood typically has a density around 0.5 g/cm³, while water is about 1 g/cm³. Because wood is less dense, it floats. A steel nail, however, has a density of roughly 7.8 g/cm³—much higher than water—so it sinks immediately.
This principle explains why large steel ships float despite being made of dense metal. Their overall density is reduced because they are designed with large hollow spaces filled with air, lowering the average density below that of water.
Buoyancy: The Invisible Force That Lifts Objects
Buoyancy is the upward force exerted by a fluid on any object immersed in it. This force opposes gravity and is central to floating behavior. The concept was first described by the ancient Greek mathematician Archimedes, who famously declared “Eureka!” upon discovering the principle while investigating a crown’s purity.
Archimedes’ Principle states: *The buoyant force on an object submerged in a fluid is equal to the weight of the fluid displaced by the object.* If this upward force is greater than or equal to the object’s weight, the object floats.
Consider a beach ball pushed underwater. It displaces a volume of water that weighs more than the ball itself, resulting in a strong upward buoyant force. Release it, and it rockets to the surface. In contrast, a stone displaces water that weighs less than the stone, so the buoyant force is insufficient to counteract gravity—it sinks.
“Any body completely or partially submerged in a fluid is acted upon by an upward force equal to the weight of the fluid displaced.” — Archimedes, c. 250 BCE
Factors That Influence Buoyancy and Density
Several variables affect whether an object floats or sinks, even if its material composition remains unchanged:
- Shape and Volume: A lump of clay sinks, but reshaped into a bowl, it can displace more water and float due to increased volume without added mass.
- Fluid Density: Saltwater is denser than freshwater due to dissolved salts. This is why it’s easier to float in the ocean than in a lake.
- Temperature: Heating a fluid usually decreases its density. Warm water rises in cooler water, affecting buoyancy in natural systems like oceans.
- Compression: Gases are highly compressible. Submarines use ballast tanks to take in or expel water, changing their overall density to dive or surface.
Real-World Example: The Dead Sea Float
One of the most striking examples of fluid density in action is the Dead Sea. With salt concentrations over ten times that of regular seawater, its density exceeds 1.24 g/cm³. Human bodies, averaging about 0.98 g/cm³, become naturally buoyant. Visitors effortlessly float on the surface, unable to sink even if they try. This phenomenon illustrates how altering the fluid—not the object—can dramatically change floating behavior.
Do’s and Don’ts of Density and Buoyancy Experiments
| Do | Don’t |
|---|---|
| Use containers with clear sides to observe displacement | Assume all metals sink (e.g., some alloys like sodium-potassium float on oil) |
| Measure mass with a digital scale for accuracy | Ignore air pockets in objects—they reduce effective density |
| Try materials of varying densities: cork, plastic, aluminum, iron | Drop heavy objects into glass containers—risk of breakage |
| Compare floating in tap water vs. saltwater solutions | Forget temperature effects—warm fluids are less dense |
Step-by-Step Guide: Test Density at Home
You don’t need a lab to explore these principles. Here’s how to conduct a simple experiment to see density and buoyancy in action:
- Gather materials: A clear jar, water, food coloring (optional), and small objects (cork, grape, coin, plastic toy, rubber ball).
- Fill the jar with water, leaving space at the top.
- Predict each object’s behavior: Will it float or sink? Record your guesses.
- Test one object at a time, gently placing it into the water.
- Observe and record results. Discuss why each behaved as it did based on density.
- Enhance the test: Dissolve 3–4 tablespoons of salt in warm water, cool it, and repeat. Note differences.
This hands-on approach helps internalize abstract concepts through direct observation—especially effective for students and curious minds of all ages.
Frequently Asked Questions
Can a heavy object float?
Yes, if its overall density is less than the fluid it’s in. Ships weigh thousands of tons but float because their design includes air-filled compartments, reducing average density below that of water.
Why does ice float on water?
Water is unusual: it expands when it freezes, making ice less dense than liquid water. At 0°C, ice has a density of about 0.917 g/cm³, compared to 0.9998 g/cm³ for cold water. This unique property insulates lakes and rivers, allowing aquatic life to survive winters beneath the ice.
Does size affect whether something floats?
No, not directly. Size alone doesn’t determine flotation—density does. A tiny pebble sinks, while a massive wooden log floats. However, larger volume can help displace more water, increasing buoyant force when combined with low mass.
Practical Applications of Buoyancy and Density
These principles aren’t just academic—they power real-world technologies and natural processes:
- Marine Engineering: Shipbuilders calculate displacement and center of buoyancy to ensure stability.
- Submarines: Use ballast tanks to control density, submerging by flooding tanks with water and surfacing by expelling it with compressed air.
- Hot Air Balloons: Rely on heated air, which is less dense than cooler surrounding air, creating lift.
- Oceanography: Scientists track ocean currents influenced by density differences from temperature and salinity (thermohaline circulation).
- Medical Diagnostics: Urine specific gravity tests assess kidney function by measuring fluid density.
“Understanding buoyancy isn’t just about boats—it’s about grasping how fluids respond to matter, a cornerstone of both engineering and environmental science.” — Dr. Lena Patel, Fluid Dynamics Researcher, MIT
Conclusion: Mastering the Science of Float and Sink
Density and buoyancy are more than classroom concepts—they’re essential to navigating the physical world. From designing safer vessels to appreciating why lakes freeze from the top down, these principles offer insight into nature’s balance between weight and support. By recognizing how mass, volume, and fluid interaction determine flotation, we gain a deeper appreciation for both everyday wonders and advanced technology.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?