Have you ever noticed that fish tend to gather in cooler parts of a lake or stream? Or why aquatic life struggles during heatwaves? The answer lies in a fundamental principle of chemistry: warm water holds less dissolved oxygen than cold water. This simple fact has profound implications for ecosystems, fisheries, and even climate change. In this article, we’ll break down the science behind this phenomenon in clear, accessible terms — no advanced degree required.
The Basics of Dissolved Oxygen
Oxygen isn’t just in the air we breathe; it also dissolves into water. This dissolved oxygen (DO) is essential for aquatic organisms like fish, insects, and even beneficial bacteria. Unlike land animals, these creatures rely on extracting oxygen directly from the water through gills or other specialized structures.
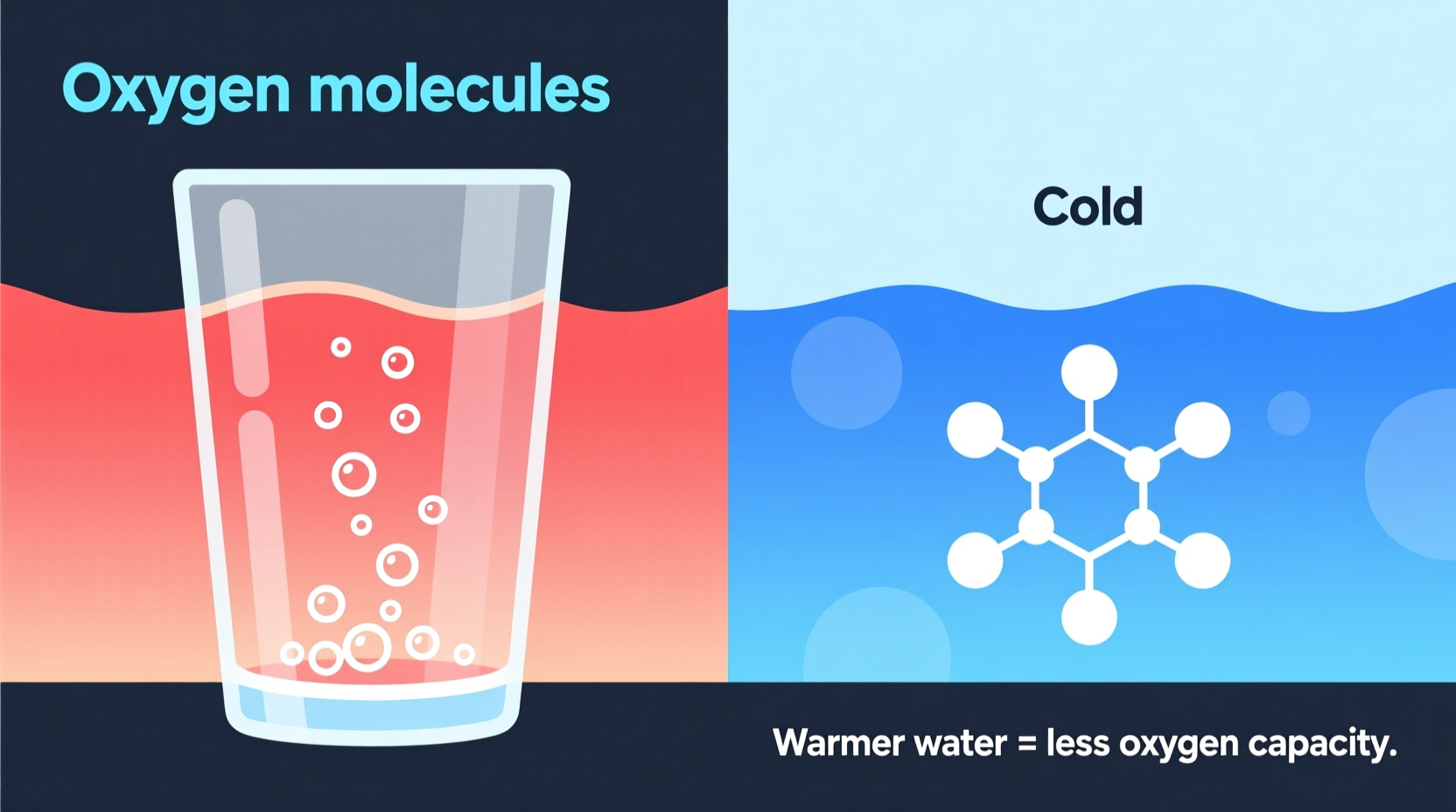
The amount of oxygen that can dissolve in water depends on several factors, but one of the most influential is temperature. As water warms up, its ability to hold oxygen decreases. This inverse relationship is predictable and measurable — and it’s rooted in basic molecular behavior.
How Temperature Affects Molecular Motion
To understand why warm water holds less oxygen, consider what happens at the molecular level. Water molecules are constantly moving. When water is cold, these molecules move slowly and are packed more closely together. This dense, calm environment makes it easier for oxygen molecules from the atmosphere to enter and remain trapped in the water.
As water heats up, the molecules gain energy and move faster. They vibrate, collide, and spread apart. This increased motion creates more space between water molecules, but it also makes the environment more chaotic. Oxygen molecules find it harder to stay dissolved because the energetic water molecules effectively \"bump\" them out of solution and back into the air.
“Think of warm water like a crowded, noisy party — gases don’t want to stick around for long.” — Dr. Lena Patel, Aquatic Biogeochemist, University of Maine
Solubility and Henry’s Law
The scientific principle governing gas solubility in liquids is known as Henry’s Law. It states that the amount of gas that dissolves in a liquid is directly proportional to the partial pressure of that gas above the liquid — but only at a constant temperature.
In practical terms, this means that under the same atmospheric conditions, cold water will absorb and retain more oxygen than warm water. For example:
| Water Temperature (°C) | Dissolved Oxygen (mg/L) |
|---|---|
| 0°C (ice-cold) | 14.6 mg/L |
| 10°C (cool) | 11.3 mg/L |
| 20°C (room temp) | 9.1 mg/L |
| 30°C (warm) | 7.5 mg/L |
This table shows how oxygen solubility drops significantly as temperature rises. At near-freezing temperatures, water can hold nearly twice as much oxygen as it can at tropical levels.
Real-World Impacts: Fish Kills and Algal Blooms
The connection between warm water and low oxygen isn’t just theoretical — it plays out dramatically in natural systems. One common consequence is summer fish kills.
During prolonged heatwaves, surface waters in lakes and ponds warm rapidly. Because warm water is less dense, it forms a layer on top that doesn’t mix easily with cooler, deeper water. This process, called thermal stratification, traps oxygen-poor water at the bottom.
At the same time, warmer temperatures accelerate the metabolism of aquatic organisms, increasing their oxygen demand. Bacteria breaking down dead algae or organic matter also consume more oxygen in warm conditions. The result? A dangerous mismatch: higher oxygen needs but lower supply.
Mini Case Study: Lake Erie Summer Die-Off
In July 2022, a stretch of shoreline along western Lake Erie saw hundreds of dead fish wash up after a week of 35°C (95°F) weather. Investigation revealed that surface temperatures had reached 28°C, reducing oxygen levels below 3 mg/L — critically low for species like walleye and perch. Thermal stratification prevented oxygen replenishment from deeper layers, while decaying algal blooms further depleted available oxygen. Scientists concluded that the combination of high temperature and nutrient runoff created a lethal environment.
This event highlights how climate trends — rising air temperatures and more frequent heatwaves — are increasing stress on freshwater ecosystems.
Practical Tips for Managing Oxygen Levels
Whether you're managing a backyard pond, an aquarium, or involved in conservation efforts, understanding the temperature-oxygen relationship helps you take proactive steps.
Checklist: Maintaining Healthy Oxygen Levels in Water Systems
- Monitor water temperature regularly, especially in summer.
- Use a dissolved oxygen test kit for ponds or aquaculture setups.
- Aerate water using fountains, bubblers, or cascading filters.
- Avoid overstocking fish in tanks or ponds.
- Control nutrient inputs (like fertilizer runoff) to prevent algal blooms.
- Preserve riparian shade by planting trees along stream banks.
- Limit organic debris buildup (leaves, grass clippings) in water bodies.
Do’s and Don’ts: Temperature and Oxygen Management
| Action | Do | Don’t |
|---|---|---|
| Aeration | Use surface agitators or diffusers in warm months | Rely solely on passive diffusion in stagnant water |
| Shading | Plant native trees along water edges | Cut down riparian vegetation |
| Monitoring | Test DO and temperature daily during heatwaves | Assume water looks fine just because it's clear |
| Nutrient Control | Use buffer zones to filter runoff | Apply fertilizers near water bodies |
FAQ: Common Questions About Warm Water and Oxygen
Why can’t fish survive in warm water if there’s still some oxygen?
Fish require a minimum threshold of dissolved oxygen (typically 5–6 mg/L for most species). While warm water may still contain oxygen, it often falls below this critical level. Additionally, warm water increases fish metabolism, meaning they need more oxygen just when less is available — leading to stress or suffocation.
Can cold water have too much oxygen?
Yes, but it’s rare. Extremely high oxygen levels (above 12 mg/L) can cause gas bubble disease in fish, similar to “the bends” in divers. However, this usually only occurs due to artificial supersaturation, such as from rapid aeration in hatcheries, not natural conditions.
Does saltwater behave the same way?
Yes, the same principle applies: warmer saltwater holds less oxygen than colder saltwater. However, saltwater naturally holds less oxygen than freshwater due to higher salinity. Combined with warming oceans, this contributes to expanding “dead zones” in coastal areas.
Conclusion: A Small Change with Big Consequences
The fact that warm water holds less oxygen might seem like a minor detail, but it’s a linchpin in aquatic ecology. From backyard ponds to global oceans, temperature-driven changes in oxygen availability affect biodiversity, food webs, and ecosystem resilience. As climate change pushes water temperatures upward, understanding this relationship becomes not just academic — it’s urgent.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?