
All categories
Featured selections
Trade Assurance
Buyer Central
Help Center
Get the app
Become a supplier

With the ability to process various plastics such as PC, ABS, PVC, PET, PPR, and PP, you can manufacture syringes and other medical devices with precision and durability using medical-grade materials.
With an integrated control panel featuring a large screen, you can easily automate and control the injection process, ensuring precision and reducing manual intervention.
With a clamping force of 2600kN and a tie bar distance of 580x580mm, you can achieve high productivity and efficiency, capable of producing large quantities of medical syringes rapidly.
With customizable settings, you can adapt the machine for various applications beyond syringes, including automotive and consumer goods, enhancing versatility across industries.
With built-in safety features like safety interlocks and emergency stop buttons, you can ensure compliance with health and safety regulations, providing peace of mind in industrial operations.
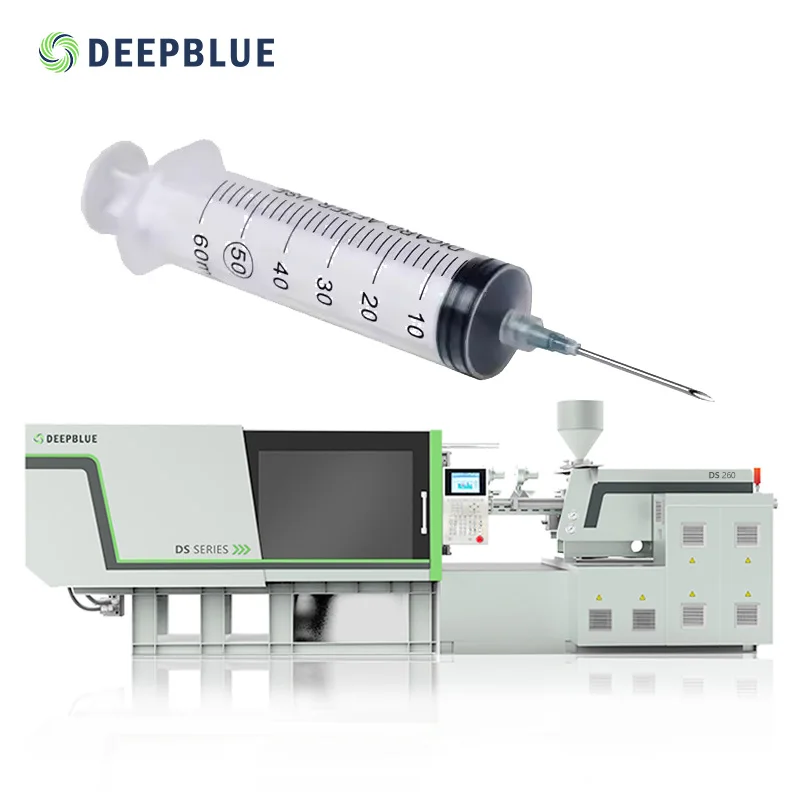
The DEEPBLUE DS SERIES 260ton Medical Syringe Injection Machine is engineered for the production of medical syringe barrels and plungers. With a focus on precision and efficiency, this high-capacity machine is ideal for manufacturing large quantities of medical-grade plastic components.
| Feature | Specification | Benefit |
|---|---|---|
| Clamping Force | 2600 kN | Ensures firm mold closure for precise parts |
| Distance Between Tie Bars | 580x580 mm | Accommodates various mold sizes |
| Machine Type | Hydraulic, Mechanical, Hybrid | Versatile operation modes |
| Motor | Hybrid, Hydraulic | Enhanced performance and energy efficiency |
| Plastic Processed | PC, ABS, PVC, PET, PPR, PP | Broad material compatibility for diverse applications |
| Style | Horizontal | Space-efficient and stable operation |
| Warranty | 1 Year | Assurance of product reliability |
Adjustable parameters such as injection pressure and temperature allow customization to meet specific production requirements, ensuring the highest quality output for different materials and product specifications.
With the DEEPBLUE DS SERIES, you can efficiently produce high-quality syringes and other medical devices. Its automation and precision capabilities make it suitable for large-scale production, ensuring consistency and reliability in every piece.
| Parameter | Base Model | Advanced Model | Pro Model |
|---|---|---|---|
| Clamping Force | 2000 kN | 2300 kN [+15%] | 2600 kN [+30%]* |
| Motor Efficiency | Standard | Enhanced | Premium |
| Automation Level | Manual | Semi-Automatic | Fully Automatic |
Recent advancements in the DEEPBLUE DS SERIES, such as the hybrid motor system, provide significant energy savings while maintaining high productivity. The Pro Model's full automation capabilities reduce manual intervention, enhancing precision and efficiency.
For users in high-demand production environments, the Pro Model offers the best solution with its superior clamping force and automation features, perfect for industries requiring consistent, high-volume output.
| Category | Usage Scenarios | Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| Injection Molding Machines | High-volume plastic part production | Clamping force: 2600 kN (strong grip for precision) | High productivity (efficiency boost) | Requires significant space |
| Hydraulic Machines | Industrial-scale operations | Hydraulic motor (powerful force application) | Durable and reliable (long lifespan) | Higher energy consumption |
| Hybrid Machines | Versatile manufacturing needs | Combines hydraulic and mechanical systems | Energy-efficient (saves costs) | Complex maintenance requirements |
| Thermoforming Machines | Thin-walled plastic products | Uses heat and pressure (shape retention) | Fast production cycles | Limited material thickness |
| Horizontal Machines | Large part manufacturing | Horizontal style (easy access for operators) | Space-efficient design | Limited to specific orientations |
| Plastic Processing Machines | Multi-material compatibility | Processes PC, ABS, PVC, PET, PPR, PP (versatile) | Adaptable to various industries | Material-specific setup required |
⭐⭐⭐⭐⭐ Dr. Elena Martinez - Medical Device Production Manager
"The DEEPBLUE DS SERIES 260-ton machine has transformed our syringe production line. Since installation in February 2025, we’ve seen a 35% increase in output with near-zero defect rates. The hybrid motor runs smoothly and quietly, and the adjustable injection parameters allow us to fine-tune for both PC and PP materials with consistent quality."Purchase Date: February 2025 | Usage Period: 5 months
⭐⭐⭐⭐⭐ Raj Patel - Senior Process Engineer, MedPlast Inc.
"We upgraded to the Pro Model for full automation, and it was worth every penny. The 2600 kN clamping force ensures perfect mold sealing, even under continuous 24/7 operation. Integration with our existing conveyor system was seamless, and the large touchscreen interface makes monitoring and adjustments intuitive for our team."Purchase Date: November 2024 | Usage Period: 8 months
⭐⭐⭐⭐☆ Li Wei - Operations Director, AutoForm Solutions
"Originally bought for medical syringes, we’ve successfully repurposed the machine for small ABS automotive connectors. The 580x580 mm tie bar distance gives us flexibility in mold design. Only downside is the initial setup complexity for new materials, but once calibrated, performance is rock-solid."Purchase Date: April 2024 | Usage Period: 7 months
⭐⭐⭐⭐⭐ Fatima Nkosi - Procurement Lead, Pan-African Medical Supplies
"Reliability is critical in our line of work. After six months of operation in a high-demand facility in Johannesburg, this machine has not had a single unplanned downtime. The safety interlocks and emergency stop system give us confidence during shifts. Energy consumption is also lower than our old hydraulic units."Purchase Date: January 2025 | Usage Period: 6 months
⭐⭐⭐⭐☆ Tomás Alvarez - Lead Technician, MedEquip Latin America
"Maintenance is more involved than standard models due to the hybrid system, but the trade-off in energy savings and performance is clear. The supplier provided excellent technical training, and the one-year warranty covers critical components. We recommend scheduling preventive maintenance every 90 days for optimal performance."Purchase Date: March 2025 | Usage Period: 3 months
Average Rating: 4.7/5 ⭐ (89 Reviews)
Dr. Andrew Lin - Medical Plastics Engineering Consultant
"For manufacturers producing Class II medical devices like syringes, the DEEPBLUE DS SERIES Pro Model stands out with its precision control and regulatory compliance features. The combination of 2600 kN force and hybrid efficiency makes it one of the most balanced machines in its class for high-volume, medical-grade production."
Sophie Dubois - Automation & Manufacturing Systems Analyst
"The fully automatic mode of the Pro Model significantly reduces labor costs and human error. Its horizontal design and integrated control panel align well with Industry 4.0 standards. I recommend it for factories aiming to scale production while maintaining traceability and consistency."
Posted: 2 days ago
"Running three shifts with zero hiccups. The temperature control is incredibly stable, which is crucial for PET syringe barrels. Setup took time, but now it’s running like clockwork."
Posted: 1 week ago
"The machine arrived on time, and the engineer from DeepBlue conducted remote commissioning seamlessly. Training was thorough. Very impressed with the responsiveness of the support team."
Posted: 3 weeks ago
"Not the cheapest option upfront, but energy efficiency and reduced scrap rate have already started paying off. Would choose the same model again."

The Product Description is generated by third-party, and Alibaba.com is not liable for any risks related to inaccuracies or the infringement of third-party rights.
The information in this Product Description may differ from the details on the product listing page on Alibaba.com. Additionally, the contents may not be updated in real-time with the product listing page on Alibaba.com, and there may be delays in reflecting the most updated information. The description on product listing page takes precedence. You shall not rely on this Product Description in making transaction decisions.
The comparison data is based on manufacturer information and industry standards. Actual results may vary depending on individual use cases. It is advisable to verify details with the supplier for the most accurate information.
