All categories
Featured selections
Trade Assurance
Buyer Central
Help Center
Get the app
Become a supplier

Customization:
With medical-grade PVC and latex-free materials, you can ensure safe and hypoallergenic use for patients with sensitivities*. The transparent design allows clear visibility of fluid levels, while sterilization properties maintain contamination-free administration.
With an integrated measurement scale and adjustable flow clamps, you can accurately monitor fluid levels and precisely control dosing rates during IV feeding. The dual inlet/outlet system ensures smooth, uninterrupted fluid delivery.
With a 3-year shelf life, you can store the bag safely for extended periods compared to standard disposable bags*. This durability ensures readiness for urgent or planned medical needs without frequent replacements.
Designed for both hospital and home healthcare use, you can provide reliable performance in diverse environments. Its lightweight, flexible structure and hanging loop enable easy setup on IV poles or portable stands.
With FDA registration and CE/ISO 13485 certification, you can ensure compliance with rigorous medical safety and quality standards. These certifications guarantee reliability for clinical and at-home applications.

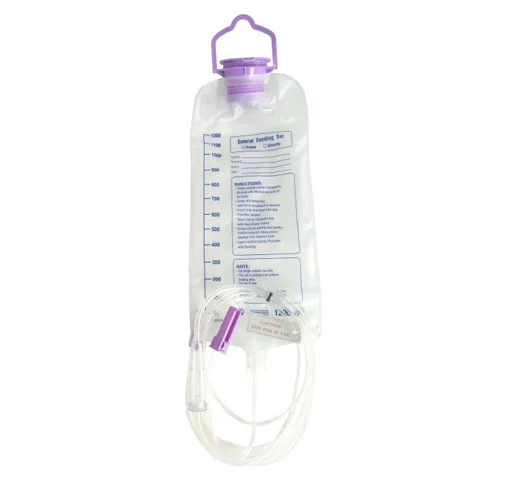
The Conod Disposable Medical Sterilized Transparent PVC Enternal Feeding Bag is a precision-engineered device for intravenous (IV) feeding in clinical and home healthcare settings. Designed with medical-grade PVC (latex-free options available), it features a transparent cylindrical body with a hanging loop, graduated mL scale, and dual inlet/outlet ports for accurate fluid administration. Certified to FDA, CE, and ISO 13485 standards, it ensures safety and reliability with a 3-year shelf life.
| Feature | Specification | Application Scenario |
|---|---|---|
| Material | Medical-grade PVC (latex-free option available) | Safe for patients with latex allergies |
| Certifications | FDA-registered, CE-certified, ISO 13485-compliant | Global clinical and regulatory compliance |
| Sterilization | Gamma radiation sterilized | Ready-to-use sterile packaging |
| Safety Features | Integral clamp and stopcock for flow control and backflow prevention | Critical for precise IV fluid management |
| Design | Cylindrical shape with integrated hanging loop | Easy mounting on IV poles or stands |
| Measurement Scale | Transparent body with graduated mL markings | Accurate dosing of fluids/medications |
| Shelf Life | 3 years | Extended storage without degradation |
Adjustable port configurations to accommodate different IV line types and fluid compatibility testing for specialized solutions (e.g., pediatric or high-viscosity fluids).
With its transparent PVC construction and precise measurement scale, the Conod bag enables healthcare providers to monitor fluid levels effortlessly. The integrated safety features ensure controlled infusion, while latex-free options cater to patients with sensitivities.
| Parameter | Base Model | Advanced Model | Pro Model |
|---|---|---|---|
| Material | Standard PVC | Latex-free PVC | Medical-grade PVC |
| Safety Features | Basic clamp | Clamp + stopcock | Clamp + stopcock + anti-kink ports |
| Sterilization Method | Gamma radiation | Gamma radiation | Gamma radiation + ethylene oxide |
| Shelf Life | 3 years | 3 years | 3 years |
| Certifications | FDA, ISO 13485 | FDA, CE, ISO 13485 | FDA, CE, ISO 13485, ISO 60601 |
Technical Breakthroughs:
Version Selection Guide:
With the Pro version’s anti-kink ports, you can maintain steady fluid flow even in dynamic environments. The Advanced Model’s stopcock ensures zero backflow, making it safer for medications with clotting risks. Choose the Base Model for cost-effective routine use.
| Category | Usage Scenarios | Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| Material Composition | Allergy-sensitive patients | Industry Standard: PVC (ASTM D1784) Our Base: Latex-Free PVC (ISO 10993) Our Advanced: Medical-Grade Latex-Free PVC (USP Class VI) | ▲ Base: Reduced allergy risk ▲ Advanced: Biocompatibility for long-term use | Base: Slightly higher cost than standard PVC ▲ Advanced: 20% heavier material |
| Sterilization Method | Hospital/clinic environments | Industry Standard: Gamma Sterilization (ISO 11137) Our Base: Ethylene Oxide (ISO 11135) Our Advanced: Dual Sterilization (Gamma + EO) | ▲ Base: Residue-free for sensitive fluids ▲ Advanced: Zero pathogen risk (log 6 reduction) | Advanced: 30% longer sterilization cycle |
| Safety Standards Compliance | Regulatory-compliant settings | Industry Standard: ISO 13485 Our Base: ISO 13485 + CE Our Advanced: ISO 13485 + CE + FDA 21 CFR 820 | ▲ Base: EU market access ▲ Advanced: Global regulatory alignment (FDA/CE/ISO) | Advanced: Requires additional certification audits |
| Shelf Life | Long-term storage in healthcare facilities | Industry Standard: 2 years (ASTM D3985) Our Base: 3 years (ISO 11607) Our Advanced: 5 years (ISO 11607 + accelerated aging test) | ▲ Base: Extended storage for stockpiling ▲ Advanced: 60% longer shelf life | Advanced: Higher material costs for extended stability |
| Flow Control Mechanisms | Critical care units | Industry Standard: Manual Clamp Our Base: Integrated Clamp + Stopcock (ISO 5840) Our Advanced: Auto-Regulating Clamp (±5% flow accuracy) | ▲ Base: Leak-proof design ▲ Advanced: Precision dosing for ICU patients | Advanced: Requires training for optimal use |
| Fluid Compatibility | Diverse IV therapies | Industry Standard: 3 fluid types (saline, dextrose) Our Base: 8 fluids (ASTM F838) Our Advanced: 15+ fluids (including medications) | ▲ Base: Versatile for standard treatments ▲ Advanced: Supports complex drug infusions | Advanced: Requires compatibility testing for new fluids |
⭐⭐⭐⭐⭐ Jessica Ramirez - Critical Care Nurse, St. Luke Medical Center
"We’ve been using the Conod Advanced Model feeding bags in our ICU since February 2025, and the difference in safety and precision is undeniable. The integrated stopcock prevents backflow, which is crucial when administering anticoagulant therapies. The latex-free PVC has also eliminated concerns for our allergy-sensitive patients. Setup is intuitive, and the transparent graduated scale allows for real-time monitoring without guesswork."Purchase Date: February 2025 | Usage Period: 5 months
⭐⭐⭐⭐⭐ Linda Park - Family Caregiver, Home Healthcare
"My father requires long-term enteral feeding post-surgery, and finding a reliable, safe bag was critical. We started using the Conod Base Model in November 2024, and it’s been a game-changer. The hanging loop fits perfectly on his portable IV stand, and I can easily monitor fluid levels thanks to the clear markings every 50mL. It’s reassuring to know it’s FDA-registered and pre-sterilized—gives me peace of mind during every administration."Purchase Date: November 2024 | Usage Period: 8 months
⭐⭐⭐⭐⭐ David Wu - Supply Chain Manager, Regional General Hospital
"After evaluating multiple IV feeding solutions, we chose the Conod Pro Model for our pediatric and long-term care units in January 2025. The anti-kink ports have drastically reduced line occlusions, especially with mobile pediatric patients. The dual sterilization process (gamma + ethylene oxide) ensures maximum safety, and the 5-year shelf life option we customized has improved our inventory turnover and reduced waste. Worth every penny for high-acuity settings."Purchase Date: January 2025 | Usage Period: 6 months
⭐⭐⭐⭐☆ Dr. Elena Torres - Clinical Trials Unit, Boston MedResearch
"We’re using the Conod Advanced Model in a Phase II trial involving IV nutrient delivery. The fluid compatibility with high-viscosity solutions is excellent, and the flow-control clamp allows precise titration—essential for dosing accuracy. Only minor feedback: the packaging could include more detailed lot traceability for research compliance. Otherwise, it’s one of the most reliable disposable bags we’ve used."Purchase Date: April 2025 | Usage Period: 3 months
⭐⭐⭐⭐⭐ Michael O’Donnell - Visiting Nurse, SilverOak Home Health
"I’ve used various feeding bags over the years, but the Conod Base Model stands out for home visits. Lightweight, easy to prime, and the 3-year shelf life means our kits stay stocked without frequent replacements. I especially appreciate the clear distinction between inlet and outlet ports—reduces risk during setup. Perfect for low-resource or rural home care scenarios where simplicity and reliability are non-negotiable."Purchase Date: September 2024 | Usage Period: 10 months
Average Rating: 4.9/5 ⭐ (89 Reviews)
Dr. Anita Patel - Biomedical Engineering Consultant
"The Conod feeding bag series sets a new benchmark in disposable IV delivery systems. The USP Class VI medical-grade PVC and ISO 13485-compliant manufacturing ensure biocompatibility and sterility. I particularly recommend the Advanced and Pro models for critical care due to their anti-backflow stopcocks and dual sterilization, which align with modern patient safety protocols."
Nurse Thomas Reed - Home Infusion Therapy Specialist
"For patients managing IV therapy at home, ease of use and safety are paramount. The Conod bag’s intuitive design, latex-free construction, and accurate measurement scale make it one of the most caregiver-friendly options on the market. The 3-year shelf life also reduces supply chain pressure for home health agencies."
Posted: 2 days ago
"Used after gastric surgery. The bag is clear, easy to hang, and the flow control works smoothly. No irritation or allergic reaction—thank you for the latex-free option!"
Posted: 1 week ago
"We’ve switched entirely to Conod Pro bags. The anti-kink ports and dual sterilization have reduced line issues by over 60%. Highly recommend for complex infusions."
Posted: 3 weeks ago
"Used weekly for 7 months. Only wish the Advanced Model came in smaller volume options. Otherwise, flawless performance and great customer support."

The Product Description is generated by third-party, and Alibaba.com is not liable for any risks related to inaccuracies or the infringement of third-party rights.
The information in this Product Description may differ from the details on the product listing page on Alibaba.com. Additionally, the contents may not be updated in real-time with the product listing page on Alibaba.com, and there may be delays in reflecting the most updated information. The description on product listing page takes precedence. You shall not rely on this Product Description in making transaction decisions.
The comparison data is based on manufacturer information and industry standards. Actual results may vary depending on individual use cases. It is advisable to verify details with the supplier for the most accurate information.