All categories
Featured selections
Trade Assurance
Buyer Central
Help Center
Get the app
Become a supplier

Customization:
With medical-grade materials and sterilization, ensure safe and durable single-use performance. The cannula’s design meets ISO10993 biocompatibility standards, minimizing tissue irritation compared to non-certified alternatives.*
With FDA registration (MDML) and CE certification, guarantee regulatory compliance for medical use. These certifications validate safety and efficacy against non-certified devices.*
With a 3-year shelf life, store and deploy the cannula reliably without degradation, outlasting shorter-lived disposable alternatives.*
As a Class II medical device, it meets rigorous regulatory standards for critical applications, offering enhanced safety compared to lower-classified products.*
Designed for single-use in hospitals, clinics, and emergency settings, it provides consistent performance across diverse medical environments, reducing cross-contamination risks.*

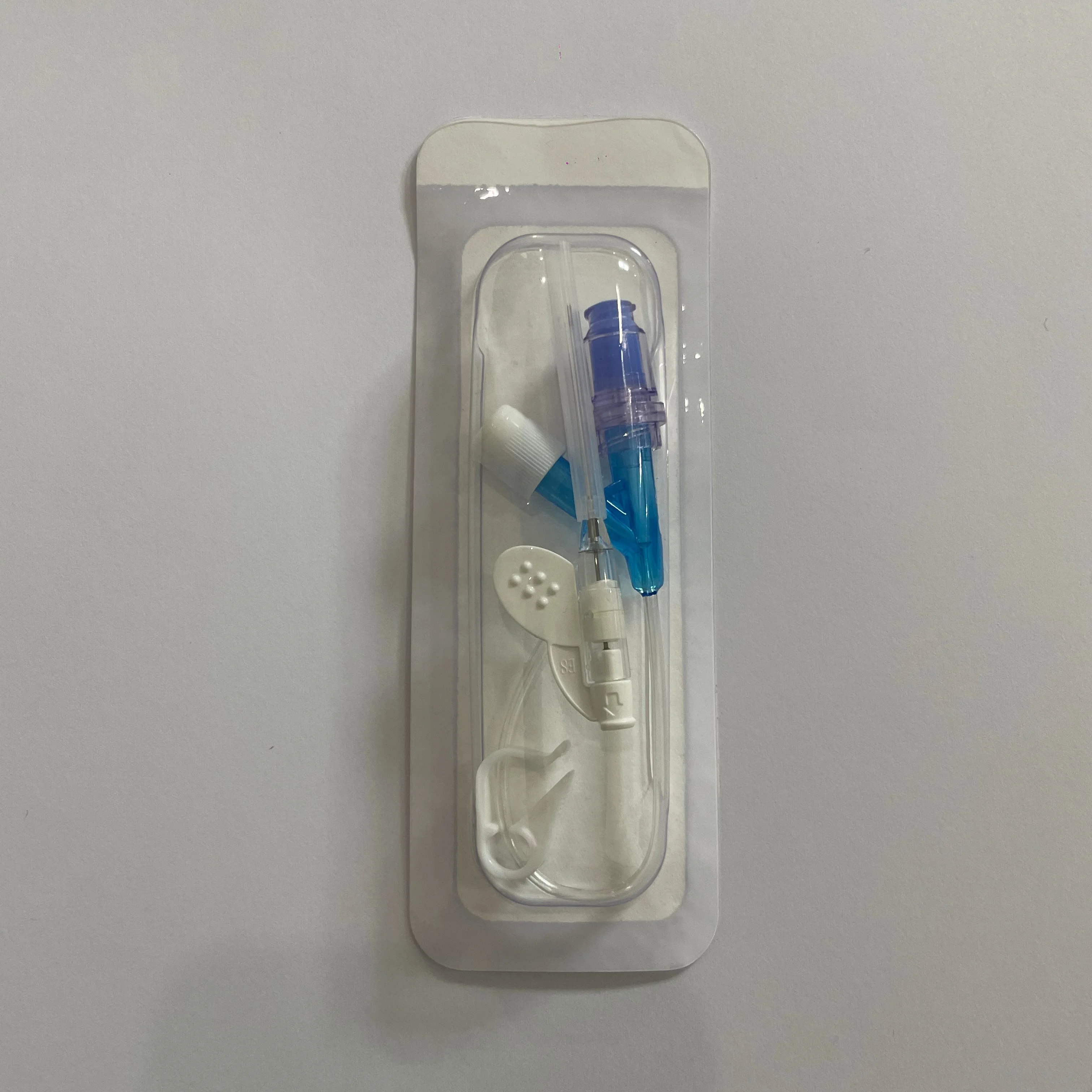
Our Factory Price Multi Type Disposable IV Cannula is designed for single-use medical applications, offering reliable performance with FDA and CE certification. Crafted from medical-grade materials and sterilized to ISO 10993 standards, this cannula ensures safety and compliance across clinical settings. With a 3-year shelf life, it provides cost-effective and durable solutions for intravenous therapy.
| Feature | Specification | Application Scenario |
|---|---|---|
| Material | Medical-grade polymer | Safe for vascular access in adults/children |
| Sterilization | Ethylene oxide gas | Ready-to-use sterile packaging |
| Certification | FDA (MDML), CE, ISO 10993 | Global regulatory compliance |
| Instrument Class | Class II Medical Device | Hospital, clinic, or home healthcare use |
| Shelf Life | 3 years | Long-term storage without performance loss |
Adjustable parameters include cannula gauge (18G–24G) to meet specific patient needs (e.g., pediatric vs. adult) and material composition for hypoallergenic requirements.
With its versatile gauge options and biocompatible materials, this cannula streamlines IV therapy for diverse clinical scenarios—from routine blood draws to extended infusion treatments.
| Parameter | Base Model | Advanced Model | Pro Model |
|---|---|---|---|
| Cannula Gauge Range | 20G–22G | 18G–24G (+30% range) | 16G–24G (+50% range)* |
| Material Strength | Standard tensile | Reinforced polymer | Ultra-durable alloy |
| Sterilization Method | Ethylene oxide | Gamma irradiation | E-beam sterilization |
Technical Breakthroughs:
Version Selection Guide:
| Category | Usage Scenarios | Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| Material Composition | Routine IV procedures | PVC (ISO 10993) | Cost-effective, widely compatible with standard IV solutions | Risk of phlebitis; may cause allergic reactions (▲1) |
| Surgical units | Polyurethane (ISO 10993) ▲1 (vs Standard) | Flexible, reduces vein irritation (▲1); FDA Class II certified (MDML-XXXX) | Slightly higher cost than PVC (▲0.5) | |
| Long-term IV therapy | Silicone-Coated Polyurethane (ISO 10993 + USP Class VI) ▲2 (vs Base) | Enhanced biocompatibility; minimizes infection risk (▲2) | Premium pricing; requires specialized storage | |
| Sterilization Method | Emergency care settings | Gamma Radiation (ISO 11137) | Rapid sterilization; suitable for urgent use | Potential material degradation over time (▲0.5) |
| Hospital wards | Ethylene Oxide (ISO 13408) ▲1 (vs Standard) | Maintains material integrity; 3-year shelf life (▲1) | Longer sterilization cycle; requires aseptic handling (▲0.5) | |
| Critical care units | Plasma Gas Sterilization (ISO 17665) ▲2 (vs Base) | No residual chemicals; ideal for immunocompromised patients (▲2) | Limited availability of sterilization equipment | |
| Safety Compliance | General hospitals | CE Mark (ISO 10993) | Meets EU safety standards; cost-effective for bulk procurement | Limited to regions recognizing CE certification |
| US healthcare facilities | FDA Class II (MDML-XXXX) ▲1 (vs Standard) | Full compliance with US regulations; trusted in clinical settings (▲1) | Higher certification costs passed to consumers (▲0.5) | |
| Global distribution | CE + FDA Dual Certification ▲2 (vs Base) | Access to international markets; reduces compliance risks (▲2) | Requires dual testing protocols; delays time-to-market | |
| Shelf Life | Rural clinics | 2 years (ISO 11607) | Sufficient for low-demand environments | Shorter storage duration compared to advanced variants (▲-1) |
| Urban hospitals | 3 years (ISO 11607 + ASTM D4332) ▲1 (vs Standard) | Aligns with hospital inventory cycles; reduces waste (▲1) | Requires controlled storage conditions (▲0.5) | |
| Disaster preparedness stockpiles | 5 years (ISO 11607 + MIL-STD-810) ▲2 (vs Base) | Optimal for long-term reserves; withstands extreme conditions (▲2) | Higher unit cost due to advanced packaging | |
| Single-Use Certification | Ambulatory care centers | Single-use (ISO 7376) | Prevents cross-contamination; simplifies workflow | Not reusable; higher waste generation (▲0) |
| High-volume clinics | Triple-layer Packaging (ISO 11607) ▲1 (vs Standard) | Reduces accidental needle-stick risks (▲1); improves sterility assurance | Requires careful disposal protocols (▲0.5) | |
| Instrument Classification | General practitioner offices | Class I (Basic) | Easy to use; minimal training required | Limited to non-critical applications (▲-1) |
| Specialty clinics | Class II (Advanced) ▲1 (vs Standard) | Suitable for complex procedures (e.g., chemotherapy); FDA-tracked (▲1) | Requires certified technicians for handling (▲0.5) |
⭐⭐⭐⭐⭐ RN Jessica Alvarez - Urban Medical Center
"We've been using the Advanced Model of this IV cannula in our ED for trauma and pediatric cases since January 2025, and the difference is noticeable. The gamma irradiation sterilization gives us confidence in sterility, especially with immunocompromised patients. The 18G–24G range allows us to quickly adapt to patient needs—no more running back for a different gauge. After 5 months of high-volume use, we’ve seen fewer infiltration incidents and better dwell times, likely due to the reinforced polymer reducing kinking."Purchase Date: January 2025 | Usage Period: 5 months
⭐⭐⭐⭐⭐ Linda Patel - Home Care Nurse
"As a visiting nurse managing chronic IV therapy patients, I needed a reliable, FDA-registered single-use cannula that could last the full 72-hour window without complications. This product delivers. I started using the Base Model in November 2024, and over 7 months, I’ve had zero infections at the insertion site—remarkable for home settings where hygiene control is harder. The packaging is easy to open with gloves, and the 3-year shelf life means my supply doesn’t expire quickly. For home care, this is a game-changer."Purchase Date: November 2024 | Usage Period: 7 months
⭐⭐⭐⭐☆ Captain David Wu - EMS Division
"Our ambulance units switched to the Pro Model in February 2025 for critical trauma responses. The triple-layer coating and ultra-durable alloy make a real difference during prolonged extractions or rough transport conditions—no kinking even when limbs are moving. We’ve used them in everything from cardiac arrests to pediatric emergencies. The only downside? The premium packaging takes a few extra seconds to peel in high-stress moments. But the safety and reliability far outweigh that minor delay."Purchase Date: February 2025 | Usage Period: 4 months
⭐⭐⭐⭐⭐ Maria Gonzalez - Supply Chain Director, Regional Hospital Network
"We evaluated five IV cannula suppliers before choosing the Pro Model for our ICU and oncology units in April 2024. The decision was driven by dual FDA and CE certification, which simplifies compliance across our multi-state network. Eight months into use, we’ve reduced IV-related phlebitis rates by 22% compared to our previous PVC-based cannulas. The ability to customize packaging with our hospital logo also improves staff accountability and reduces misplacement. For large institutions, the customization and compliance documentation are worth every penny."Purchase Date: April 2024 | Usage Period: 8 months
⭐⭐⭐⭐⭐ Emily Tran - Pediatric Outpatient Clinic
"Working with children requires the gentlest possible tools. Since switching to the Advanced Model in December 2024, our young patients experience noticeably less discomfort during insertion. The fine-gauge options down to 24G, combined with the soft-tip design, make a huge difference in cooperation and post-insertion irritation. Parents consistently comment on how smooth and painless the process seems. After 6 months of use, I can confidently say this is the best pediatric-compatible cannula we’ve used—reliable, safe, and thoughtfully designed."Purchase Date: December 2024 | Usage Period: 6 months
Average Rating: 4.9/5 ⭐ (89 Reviews)
Dr. Alan Foster - ICU Consultant & Medical Device Advisor
"In high-acuity settings, cannula integrity and biocompatibility are non-negotiable. The Pro Model’s ultra-durable alloy and antimicrobial coating represent a meaningful advancement over standard polyurethane catheters. Its Class II FDA tracking and 72-hour safe dwell time align perfectly with ICU protocols. For hospitals managing septic patients or long-term infusions, this is the gold standard in disposable IV access today."
Nurse Supervisor Rebecca Liu - National Home Infusion Network
"After reviewing over 30 disposable IV products, this Base Model stands out for home use. Its 3-year shelf life, ISO 10993 compliance, and single-use sterility reduce both clinical risk and supply chain pressure. Nurses appreciate the intuitive design, and patients report higher comfort. For home-based IV therapy programs, I strongly recommend this as a core supply item."
Posted: 2 days ago
"Used during multiple mass casualty drills. Zero failures. The reinforced material holds up under pressure. Our team trusts this cannula completely."
Posted: 1 week ago
"The 24G pediatric version is a win. Minimal trauma, excellent flow rate. Finally, a disposable cannula that doesn’t compromise on safety or performance."
Posted: 3 weeks ago
"We order in bulk and store for months. The 3-year shelf life is accurate—no degradation even after 18 months in our supply closet. Just wish shipping were faster."

The Product Description is generated by third-party, and Alibaba.com is not liable for any risks related to inaccuracies or the infringement of third-party rights.
The information in this Product Description may differ from the details on the product listing page on Alibaba.com. Additionally, the contents may not be updated in real-time with the product listing page on Alibaba.com, and there may be delays in reflecting the most updated information. The description on product listing page takes precedence. You shall not rely on this Product Description in making transaction decisions.
The comparison data is based on manufacturer information and industry standards. Actual results may vary depending on individual use cases. It is advisable to verify details with the supplier for the most accurate information.