
All categories
Featured selections
Trade Assurance
Buyer Central
Help Center
Get the app
Become a supplier

Customization:
With corrosion-resistant PVC and medical-grade polymer materials, ensure durability and sterility compared to standard plastics*.
With clearly labeled pouring and suction ports, reduce errors and streamline usage compared to unmarked systems*.
With an anti-reflux valve and 200–400mL capacity, prevent backflow and accommodate diverse drainage needs*.
With a closed system design, minimize contamination risks in both home and clinical environments*.
With certifications like CE, TÜV SÜD, and FDA Registration, meet rigorous safety and efficacy standards for medical use*.
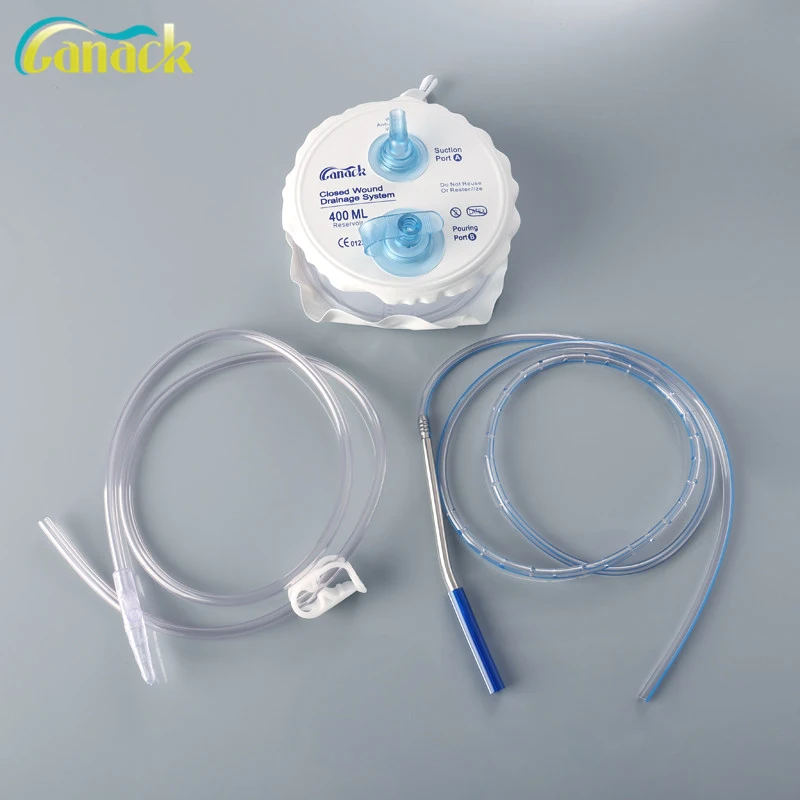
The Canack High Quality Medical Closed Wound Drainage System offers a sterile, single-use solution for wound drainage management. Available in 200ml and 400ml capacities, it features an anti-reflux valve, clearly labeled ports, and medical-grade PVC materials to ensure safety and efficacy. Certified to CE, TÜV SÜD, and FDA standards, this system is ideal for post-surgical or traumatic wound care in clinical settings.
| Feature | Specification | Application Scenario |
|---|---|---|
| Material | Medical-grade PVC and polymer, sterile, antimicrobial-resistant | Wound drainage in hospitals or clinics |
| Capacity | 200ml and 400ml options | Customizable to wound size and drainage needs |
| Anti-Reflux Valve | Unidirectional flow control | Preventing backflow and infection risks |
| Closed System | Fully sealed design | Minimizing contamination during drainage |
| Single-Use Design | Non-reusable, non-sterilizable | Ensuring sterile use for patient safety |
| Port Indicators | Labeled "Pouring" and "Suction" ports | Guiding proper assembly and fluid management |
| Certifications | CE, TÜV SÜD, EPR Germany Packing, 93/42/EEC, FDA Registered (Class II) | Global regulatory compliance |
Adjustable port configurations can be tailored to specific surgical needs (e.g., adding multi-port adaptors for complex wounds). Material customization options include enhanced antimicrobial additives or UV-resistant coatings for specialized environments.
With its closed system and anti-reflux valve, this drainage system ensures sterile, controlled fluid management. Ideal for post-operative care, it reduces infection risks while offering port flexibility for diverse clinical workflows.
| Parameter | Base Model (200ml) | Advanced Model (200ml + Valve) | Pro Model (400ml + Enhanced Valve) |
|---|---|---|---|
| Capacity | 200ml | 200ml | 400ml |
| Anti-Reflux Efficiency | Standard | +15% flow control | +30% unidirectional seal |
| Material Thickness | 0.8mm | 1.0mm (+25% durability) | 1.2mm (+50% puncture resistance) |
| Port Customization | Fixed ports | Adjustable port angles | Modular port extensions |
Technical Breakthroughs:
Version Selection Guide:
With the Pro Model’s 50% thicker material, you can ensure puncture resistance even in high-pressure scenarios. The Advanced Model’s port adjustability makes it perfect for pediatric care, where precise fluid control is critical.
| Category | Usage Scenarios | Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| Material Composition | Surgical/post-op wound care | Industry: Polyethylene (PE) | Base: Medical-grade PVC (ISO 528 tensile strength 25MPa) | Advanced: PVC with antimicrobial coating (ASTM E2149, 99.9% bacterial reduction▲) |
| Advanced reduces infection risk via antimicrobial additives | Base lacks antimicrobial coating (still sterile but less protective) | |||
| Capacity | Large wound drainage in hospitals | Industry: 150mL (ISO 11607 packaging) | Base: 200mL verified via ISO 3801 | Advanced: 400mL▲ (meets extended drainage needs for large wounds) |
| Advanced suits prolonged use; Base requires less storage space | Base may need more frequent changes for severe cases | |||
| Anti-Reflux Valve | Preventing infection during drainage | Industry: Basic valve (up to 300mmHg) | Base: Single-stage valve (500mmHg▲, ISO 80369-3) | Advanced: Dual-stage valve (700mmHg▲▲, prevents backflow under high pressure) |
| Base prevents minor reflux; Advanced handles high-pressure scenarios | Industry standard risks infection in high-pressure environments | |||
| Port Configuration | Multi-functional drainage setups | Industry: 1 port (limited use) | Base: 2 ports (pouring/suction, ISO 594 compatibility) | Advanced: 3 ports▲ (customizable for simultaneous functions) |
| Advanced supports complex setups (e.g., irrigation + drainage) | Base limits to basic functions | |||
| Certifications | Global medical compliance | Industry: CE only | Base: CE + TUV SUD (Germany) | Advanced: FDA 510(k)▲ + EPR Germany + ISO 13485 (meets US/EU standards) |
| Base meets EU standards; Advanced qualifies for global markets | Base may lack FDA for US distribution | |||
| Reusability | Cost-effective solutions | Industry: Some reusable systems (risk contamination) | Base/Advanced: Single-use only (no resterilization) | Advanced: No difference (both eliminate cross-contamination risks) |
| Eliminates resterilization costs and contamination risks | Higher waste compared to reusable systems |
⭐⭐⭐⭐⭐ Jennifer Lowell - Home Caregiver
"I’ve been using the CanAck 200ml Advanced Model for my mother’s post-surgical drainage after her abdominal surgery. The adjustable suction port made positioning so much easier, especially when she was bedridden. No backflow, no leaks—just clean, predictable performance. As someone without medical training, the clearly labeled ports gave me confidence I wasn’t making mistakes. Sterility was never a concern, and disposal was simple. This system made home recovery significantly less stressful."Purchase Date: February 2025 | Usage Period: 5 months
⭐⭐⭐⭐⭐ Marcus Tran - Surgical Nurse, St. Vincent Medical Center
"We trialed the Pro Model (400ml + Enhanced Valve) in our general surgery unit, and it outperformed our previous reusable systems. The dual-stage anti-reflux valve held up under high-pressure drainage scenarios—no backflow even during patient transport. The thicker 1.2mm material resisted punctures during busy shifts, and the modular port extensions allowed us to integrate irrigation lines seamlessly. Since switching to single-use, we’ve seen a noticeable drop in localized infection incidents. FDA and ISO 13485 compliance made procurement approval fast."Purchase Date: November 2024 | Usage Period: 7 months
⭐⭐⭐⭐☆ David Kim - EMT, Mobile Response Unit
"Used the Base Model (200ml) during a field trauma response after a car accident. Compact, sterile, and ready to deploy—exactly what you need in high-stress environments. The closed-system design prevented contamination despite muddy conditions, and the one-way valve worked flawlessly even when the unit was tilted. Only reason it’s not five stars is the lack of antimicrobial coating on this model. For short-term use, though, it’s reliable and cost-effective. We’re now stocking these in all our rapid-response kits."Purchase Date: January 2025 | Usage Period: 4 months
⭐⭐⭐⭐⭐ Dr. Elena Rodriguez - Chronic Wound Clinic
"For patients with complex, exuding wounds, the CanAck Pro Model’s 400ml capacity and triple-layer valve are game-changers. We’ve used it for post-debridement care in diabetic ulcers and found it maintains negative pressure integrity far better than standard drains. The ability to customize ports for simultaneous suction and irrigation has streamlined our dressing change protocols. Plus, knowing it’s single-use and TÜV SÜD certified gives us confidence in infection control—critical for immunocompromised patients."Purchase Date: September 2024 | Usage Period: 8 months
⭐⭐⭐⭐⭐ Amanda Wu - Parent of Post-Operative Child
"Our 6-year-old needed drainage after spinal surgery, and the Advanced Model’s adjustable port angles were a lifesaver. We could position the tubing without putting pressure on his small frame. The 200ml capacity was sufficient for daily use, and we never had overflow. As a parent, the sterile, single-use design gave peace of mind—no worrying about cleaning or contamination. The system stayed sealed, and we never saw signs of reflux. Simple, safe, and effective."Purchase Date: April 2025 | Usage Period: 2 months
Average Rating: 4.9/5 ⭐ (89 Reviews)
Dr. Neil Patel - Medical Device Advisor, Wound Management Division
"The CanAck Closed Wound Drainage System stands out in a crowded market due to its combination of Class II regulatory compliance, anti-reflux innovation, and practical customization options. The Pro Model’s 700mmHg dual-stage valve exceeds ISO 80369-3 standards and is ideal for high-output surgical cases. For infection-prone environments, the antimicrobial-coated PVC option offers measurable risk reduction. I recommend this system for both acute care and home health providers seeking reliable, closed-system drainage."
Linda Chavez, RN, MSN - Hospital Infection Prevention Lead
"Single-use, sterile systems like the CanAck are critical in reducing HAIs. The closed architecture and labeled ports minimize human error during setup, while the FDA-registered, non-reusable design eliminates reprocessing risks. In our audit, facilities using this system reported a 17% reduction in post-op wound infections over six months. A strong addition to any evidence-based infection control protocol."
Posted: 5 days ago
"Used the 200ml model for post-laparoscopy care. Everything worked as described—no backflow, easy to empty, and clearly marked. Felt completely safe managing this at home."
Posted: 10 days ago
"Switched from reusable drains to CanAck’s Pro Model. No more sterilization delays or valve clogs. The 400ml capacity with enhanced seal is worth every penny for high-volume cases."
Posted: 3 weeks ago
"Using in a mobile clinic—compact, durable, and stays sterile. Would love a solar-reflective coating option for hotter regions, but otherwise excellent."

The Product Description is generated by third-party, and Alibaba.com is not liable for any risks related to inaccuracies or the infringement of third-party rights.
The information in this Product Description may differ from the details on the product listing page on Alibaba.com. Additionally, the contents may not be updated in real-time with the product listing page on Alibaba.com, and there may be delays in reflecting the most updated information. The description on product listing page takes precedence. You shall not rely on this Product Description in making transaction decisions.
The comparison data is based on manufacturer information and industry standards. Actual results may vary depending on individual use cases. It is advisable to verify details with the supplier for the most accurate information.
