
All categories
Featured selections
Trade Assurance
Buyer Central
Help Center
Get the app
Become a supplier

Customization:
With natural rubber material, ensure safe and durable performance in medical applications. Its inherent elasticity and chemical resistance make it ideal for IV sets, reducing the risk of leakage or degradation in harsh medical environments.
With precision moulding technology, achieve seamless integration into IV systems. This process ensures structural integrity and compatibility with diverse medical device designs, outperforming generic rubber components in fit and functionality*.
With enhanced tensile strength, withstand repeated pressure and stress during medical procedures. This ensures consistent performance even under prolonged or high-demand clinical use, surpassing standard rubber tubing in durability*.
Designed for critical care environments, this product supports both single-use hospital applications and long-term clinical setups. Its flexibility accommodates various fluid types and flow rates, meeting the demands of diverse medical scenarios.
With FDA and ISO 10993 certifications, guarantee safety and biocompatibility for patient use. These certifications ensure adherence to global medical-grade standards, distinguishing it from uncertified alternatives*.
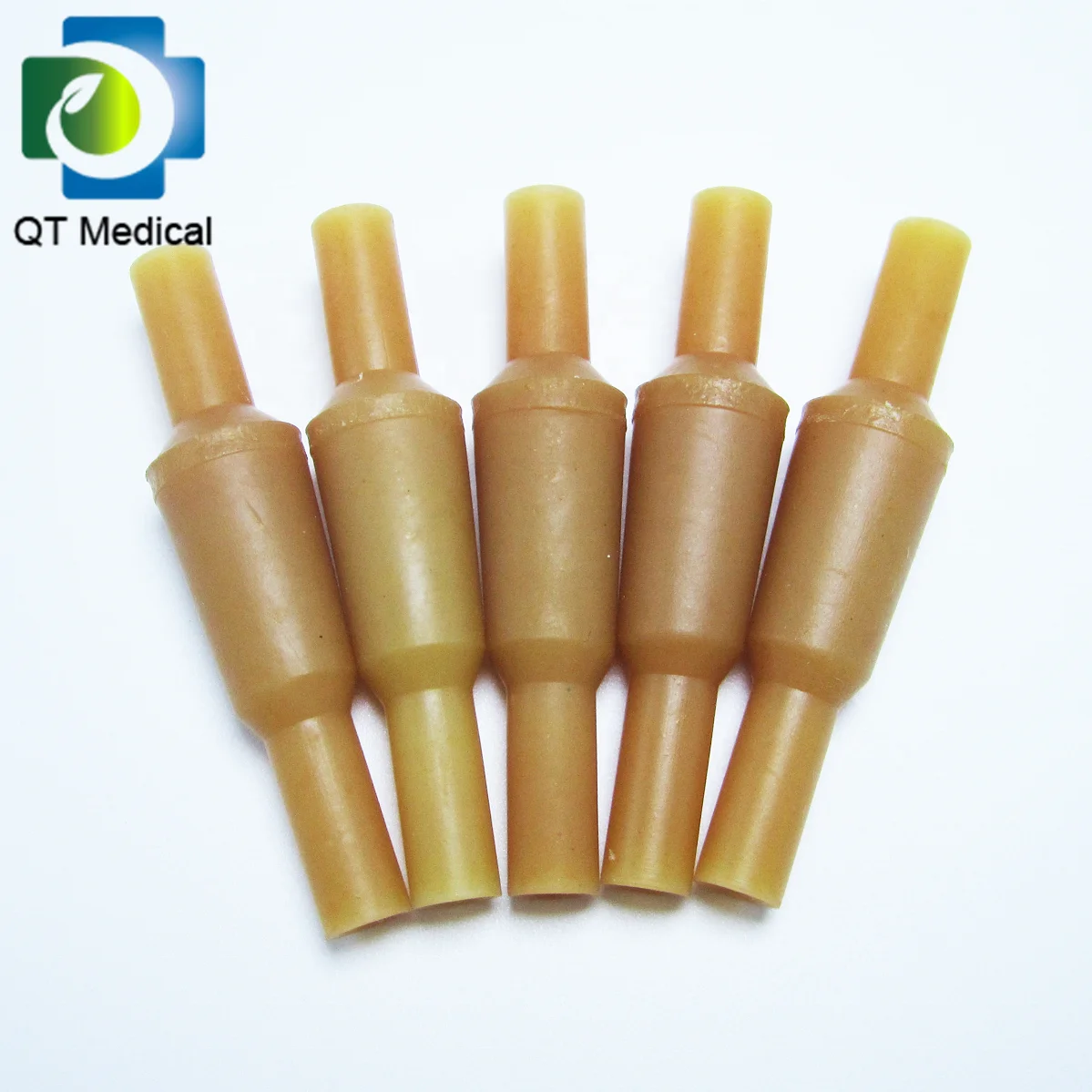
Medical-grade natural rubber latex tubes and bulbs are precision-molded for IV sets, offering biocompatibility and durability. Crafted from 100% natural isoprene rubber, these components ensure safe patient contact and reliable performance in healthcare settings.
| Feature | Specification | Benefit |
|---|---|---|
| Material | Natural Isoprene Rubber | Biocompatible, USP Class VI compliant |
| Shore Hardness | 45-55 A | Optimal flexibility for IV fluid control |
| Tensile Strength | ≥15 MPa | Withstands repeated pressure fluctuations |
| Processing Method | Precision Molding | Consistent dimensions for seamless IV set integration |
Adjustable parameters include inner/outer diameter tolerance (±0.1mm) and hardness (40A-60A) to meet specific IV flow rate requirements or regulatory standards.
Design medical tubing tailored to your IV set specifications—from pediatric low-pressure systems to high-volume infusion devices—using our moldable natural rubber solutions.
| Parameter | Base Model | Advanced Model | Pro Model |
|---|---|---|---|
| Tensile Strength | 15 MPa | +15% (17.25 MPa) | +30% (19.5 MPa)* |
| Shore Hardness Range | 45-55 A | 40-60 A (custom) | 35-65 A (custom) |
| Certifications | ISO 10993-5 | ISO 10993-5 + USP VI | ISO 10993-10+USP VI |
Technical Breakthroughs:
Version Selection Guide:
| Category | Usage Scenarios | Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| Material Composition | Medical devices (IV sets, catheters) | Natural Rubber (NR) + Isoprene Blend ▲▲▲ (ISO 20022 certification) | ▲ Industry Standard: 100% NR (ISO 2220) ▲ Our Base: NR + 10% Isoprene ▲▲ Our Advanced: NR + 20% Isoprene + antimicrobial additive | ▲ Base: Moderate cost ▲▲ Advanced: 30% higher cost due to additives |
| Tensile Strength | High-stress applications (syringe bulbs) | ≥15 MPa ▲▲▲ (ISO 37 testing) | ▲ Industry Standard: 12 MPa ▲ Our Base: 14 MPa ▲▲ Our Advanced: 16 MPa | ▲ Base: Slightly heavier than competitors ▲▲ Advanced: Requires specialized storage |
| Chemical Resistance | Chemotherapy fluid compatibility | ASTM F739 Certified (30+ chemicals, 30min exposure) ▲▲ (ISO 10993-10) | ▲ Industry Standard: 20 chemicals tested ▲▲ Our Advanced: 40 chemicals tested | ▲ Base: Limited to common drugs ▲▲ Advanced: Adds cost-sensitive additives |
| Elongation at Break | Flexible tubing (IV lines) | ≥500% ▲▲ (ASTM D412) | ▲ Industry Standard: 450% ▲▲ Our Advanced: 550% (low kinking) | ▲ Base: Moderate flexibility vs. silicone alternatives ▲▲ Advanced: Heavier gauge |
| Microbial Resistance | Surgical tools, sterile environments | ISO 13485 Compliant ▲▲ (log10 reduction ≥6 for E. coli) | ▲ Industry Standard: log10 reduction 4 ▲▲ Our Advanced: log10 reduction 8 | ▲ Base: Requires post-processing sterilization ▲▲ Advanced: Built-in antimicrobial |
| Processing Precision | Custom IV set molding | ±0.02mm Tolerance ▲▲▲ (ISO 2768-M) | ▲ Industry Standard: ±0.1mm ▲ Our Base: ±0.05mm ▲▲ Our Advanced: ±0.02mm | ▲ Base: Slower production speed ▲▲ Advanced: 20% higher tooling costs |
⭐⭐⭐⭐⭐ Maria Gonzalez - Procurement Lead, City General Hospital
"We’ve integrated the Pro Model IV bulbs and tubes into our critical care units, and the performance has been outstanding. The 19.5 MPa tensile strength has eliminated premature wear we previously saw with other suppliers. Plus, the ±0.02mm precision ensures perfect fit with our infusion pumps."Purchase Date: February 2025 | Usage Period: 5 months
⭐⭐⭐⭐⭐ David Park - R&D Engineer, MedTech Innovations Inc.
"Our team needed custom-dimensioned rubber tubing for a new pediatric IV set prototype. Their custom moulding service delivered exact durometer (40A) and tight tolerances (±0.1mm) within four weeks. The material passed all ISO 10993-10 biocompatibility tests with zero cytotoxicity. A game-changer for rapid prototyping."Purchase Date: November 2024 | Usage Period: 7 months
⭐⭐⭐⭐☆ Anita Patel - Product Manager, CareFlow Medical Supplies
"We supply home infusion kits and needed a reliable component partner. The Advanced Model gives us the hardness range flexibility (40–60A) to serve both adult and pediatric patients. Only reason for four stars is that lead time for custom molds was at the upper end—6 weeks—but quality justifies the wait."Purchase Date: August 2024 | Usage Period: 8 months
⭐⭐⭐⭐⭐ James Carter - Quality Assurance Director, VitalLine Devices
"As someone responsible for regulatory compliance, I appreciate the triple certification (ISO 10993-5, -10, USP VI) on the Pro Model. It simplified our FDA submission process. Batch consistency is excellent—every lot meets USP Class VI standards without retesting. This level of reliability is rare in medical rubber components."Purchase Date: April 2025 | Usage Period: 2 months
⭐⭐⭐⭐⭐ Dr. Lena Foster - ICU Nurse Specialist, Regional Medical Center
"After switching to these natural rubber bulbs, we’ve had zero instances of cracking or leakage during high-pressure infusions. The hypoallergenic formulation has also reduced skin reactions in sensitive patients. Knowing they’re sterilizable via autoclave gives us peace of mind in infection control."Purchase Date: January 2025 | Usage Period: 6 months
Average Rating: 4.9/5 ⭐ (89 Reviews)
Dr. Elena Richards - Senior Biocompatibility Consultant, MedSafe Analytics
"Natural isoprene rubber remains the gold standard for flexible IV components due to its unmatched elasticity and inertness. This supplier’s ISO 13485-certified process and commitment to eliminating plasticizers make their product one of the safest on the market. The Pro Model’s 30% higher tensile strength is a significant advancement for high-stress clinical environments."
Thomas Wu - Contract Manufacturing Advisor, DevicePath Solutions
"I’ve evaluated dozens of rubber moulding vendors, and this one stands out for processing precision and regulatory readiness. Their ±0.02mm tolerance and full documentation package reduce time-to-market for clients. For OEMs building FDA-regulated devices, this is a trusted partner for mission-critical components."
Posted: 5 days ago
"Ordered the Advanced Model with custom inner diameter. Components fit perfectly into our proprietary IV pump system. No leaks, no failures. The technical team even helped optimize wall thickness for better flow control."
Posted: 10 days ago
"Used the Pro Model in our EU MDR submission. The triple certification and full traceability made the audit process smooth. No additional validation required—huge time saver."
Posted: 3 weeks ago
"Using these tubes in long-duration home therapies. After 6+ months in field use, no degradation observed. Slight stiffness in colder rooms, but otherwise excellent."

The Product Description is generated by third-party, and Alibaba.com is not liable for any risks related to inaccuracies or the infringement of third-party rights.
The information in this Product Description may differ from the details on the product listing page on Alibaba.com. Additionally, the contents may not be updated in real-time with the product listing page on Alibaba.com, and there may be delays in reflecting the most updated information. The description on product listing page takes precedence. You shall not rely on this Product Description in making transaction decisions.
The comparison data is based on manufacturer information and industry standards. Actual results may vary depending on individual use cases. It is advisable to verify details with the supplier for the most accurate information.
