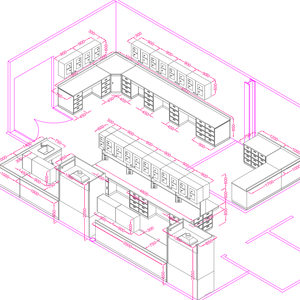
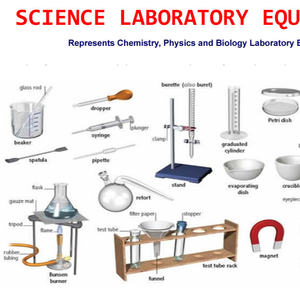
Lab Grouppen




 Top sponsor listing
Top sponsor listing




 1/2
1/2




 1/1
1/1








 1/3
1/3




 1/3
1/3




 1/3
1/3







 1/31
1/31



 1/1
1/1




 1/3
1/3





 1/3
1/3




 1/2
1/2




 1/3
1/3




 1/3
1/3







 1/12
1/12



 1/1
1/1




 1/3
1/3



 1/3
1/3





 0
0

 CN
CN





About lab grouppen
Where to Find Lab Grouppen Suppliers?
No verified suppliers for "lab grouppen" were identified in the current dataset. The term does not correspond to a recognized industrial product category in global manufacturing databases, which may indicate a spelling variation, niche application, or non-standard nomenclature. Buyers are advised to verify the correct technical designation—such as laboratory consumables, precision glassware, or specific scientific instrumentation—before initiating supplier searches.
In established industrial markets like China, Germany, and the United States, laboratory equipment and consumables production is concentrated in regions with strong scientific infrastructure and chemical processing capabilities. For example, Jiangsu and Zhejiang provinces in China host integrated clusters specializing in lab-grade plastics, borosilicate glass, and sterile packaging systems. These zones benefit from proximity to raw material suppliers and compliance-certified cleanroom facilities, enabling cost-efficient production with lead times averaging 20–35 days for standard orders.
Such ecosystems support scalable output through automated injection molding, precision extrusion, and ISO-classified assembly environments. Key advantages include adherence to international quality frameworks (e.g., ISO 17025, GLP), access to biocompatible materials (PP, PS, PETG), and compatibility with sterilization methods including autoclaving and gamma radiation. Procurement efficiency increases in regions where suppliers maintain vertical control over tooling, molding, and packaging processes within co-located facilities.
How to Choose Lab Grouppen Suppliers?
Given the absence of active suppliers under this keyword, procurement professionals should apply rigorous verification protocols when evaluating potential manufacturers for laboratory-related products:
Technical Compliance
Confirm alignment with applicable standards such as ISO 13485 for medical devices, ISO 9001 for quality management, and USP Class VI for material biocompatibility. For diagnostic or life science applications, ensure compliance with RoHS, REACH, and FDA 21 CFR Part 820 where relevant. Request test reports for critical attributes including dimensional accuracy, leak integrity, and chemical resistance.
Production Capability Audits
Assess infrastructure maturity using objective benchmarks:
- Minimum 2,000m² facility size with designated cleanroom zones (ISO 7 or higher)
- In-house mold design and CNC machining for rapid prototyping
- Automated inspection systems (e.g., vision scanning, pressure testing)
Validate claims through third-party audit reports or virtual factory walkthroughs, focusing on process validation records and batch traceability systems.
Transaction Safeguards
Utilize secure payment mechanisms such as letter of credit or escrow services until product conformity is confirmed post-delivery. Prioritize suppliers with documented export experience to regulated markets (EU, USA, Japan). Conduct sample evaluation against ASTM or DIN benchmarks prior to volume ordering—particularly for clarity, wall thickness consistency, and sterility assurance.
What Are the Best Lab Grouppen Suppliers?
No suppliers matching the product keyword “lab grouppen” are currently documented in authoritative sourcing registries. This may reflect limited market presence, incorrect terminology, or classification under alternative product codes such as laboratory disposables (HS 3926.20), measuring instruments (HS 9027), or scientific glassware (HS 7017).
Buyers seeking high-integrity laboratory supplies should instead target manufacturers with proven track records in adjacent categories. Leading-tier suppliers typically demonstrate:
- Monthly production capacity exceeding 5 million units for disposable items
- Customization support for branding, packaging configurations, and specialized geometries
- Minimum Order Quantities (MOQ) ranging from 1,000 to 10,000 pieces, depending on complexity
- Lead times of 20–40 days, inclusive of quality checks and container loading
Performance Analysis
While no direct comparisons can be made without valid supplier data, historical performance in laboratory supply chains shows that reliability correlates strongly with certification depth, facility scale, and responsiveness. Top performers maintain on-time delivery rates above 97%, respond to inquiries within 4 hours, and offer reorder rates exceeding 30% due to consistent quality and service execution. Buyers should prioritize partners with transparent documentation, export licensing, and participation in third-party inspection programs.
FAQs
How to verify lab grouppen supplier reliability?
Start by confirming the accurate product classification and technical specifications. Cross-reference company registrations with official business directories and demand copies of valid certifications issued by accredited bodies. Review transaction histories via trade platforms or customs databases to assess shipment frequency and destination diversity.
What is the average sampling timeline?
For standard laboratory consumables, expect 7–14 days for sample production. Custom molds or sterile-packaged variants may require 20–30 days. Air freight adds 5–10 days depending on destination. Allow additional time for regulatory documentation if importing into highly controlled markets.
Can suppliers ship laboratory products worldwide?
Yes, qualified manufacturers support global distribution under FOB, CIF, or DDP terms. Ensure compliance with import regulations related to medical goods, especially when shipping sterile or single-use devices. Sea freight remains optimal for full-container loads; air courier is recommended for urgent samples.
Do manufacturers provide free samples?
Sample availability depends on order commitment. Many suppliers waive fees for buyers placing initial orders above 5,000 units. Otherwise, expect to cover 30–60% of unit cost plus shipping. Refundable sample charges are common upon confirmation of bulk contracts.
How to initiate customization requests?
Submit detailed technical drawings, material preferences, and functional requirements (e.g., volume tolerance ±0.5ml, autoclave stability at 121°C). Reputable suppliers will return design feedback within 48 hours and deliver prototypes within 3 weeks. Confirm intellectual property protections before sharing proprietary designs.