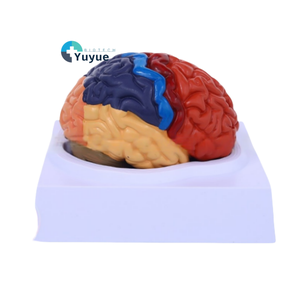
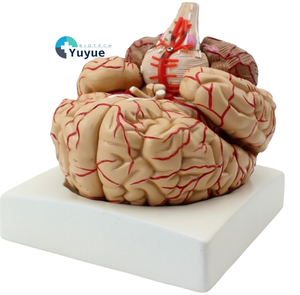
Limbic Cortex Function



 1/1
1/1






 1/48
1/48




 1/3
1/3





 1/3
1/3








 1/3
1/3





 1/3
1/3







 0
0




 1/3
1/3



 1/3
1/3







 1/13
1/13




 1/3
1/3




 1/3
1/3







 1/2
1/2




 1/3
1/3





 1/16
1/16








 1/33
1/33





 1/4
1/4
About limbic cortex function
Where to Find Limbic Cortex Function Suppliers?
The term "limbic cortex function" refers to a neurobiological concept rather than a manufactured product, representing the integrated activity of brain regions involved in emotion, memory, and behavior regulation. As such, there are no industrial suppliers of "limbic cortex function" in the traditional manufacturing or sourcing sense. Instead, research institutions, neuroscience technology developers, and biomedical equipment manufacturers provide tools and systems used to study or simulate limbic cortical activity.
Entities engaged in this domain typically operate within academic, medical device, or neurotechnology sectors. Key innovation hubs are concentrated in North America (particularly Massachusetts and California), Western Europe (Germany, Switzerland, UK), and parts of East Asia (Japan, South Korea). These regions host advanced R&D ecosystems with access to neuroimaging infrastructure, computational modeling expertise, and regulatory-compliant development frameworks for neural diagnostics and interventions.
Suppliers of related technologies—such as EEG systems, fMRI-compatible electrodes, deep brain stimulation (DBS) devices, or neuromorphic computing platforms—leverage interdisciplinary capabilities in biotechnology, microelectronics, and data science. Production is generally low-volume and high-precision, with lead times influenced by clinical validation requirements and regulatory approval cycles (e.g., FDA, CE Mark for medical devices).
How to Choose Suppliers for Limbic Cortex Function–Related Technologies?
Procurement decisions must focus on technical validity, compliance, and application-specific performance. Buyers should apply rigorous evaluation criteria when selecting partners for neuroscience-related equipment or research collaboration:
Regulatory and Quality Compliance
Prioritize suppliers holding ISO 13485 certification for medical device quality management. For diagnostic or implantable systems, confirm active CE Mark (Europe) or FDA 510(k)/PMA clearance (U.S.). Documentation should include biocompatibility testing (ISO 10993), electromagnetic compatibility (IEC 60601-1), and software validation under IEC 62304 standards.
Technical and Research Validation
Evaluate scientific credibility through peer-reviewed publications, institutional affiliations, and third-party citations of device performance. Request evidence of signal accuracy in limbic region detection (e.g., amygdala, cingulate cortex) using spatial resolution metrics (in mm³) and temporal precision (ms response). For AI-driven analysis tools, verify training datasets for neurological diversity and bias mitigation.
- Minimum requirement: Published validation studies in indexed journals (e.g., NeuroImage, Brain Stimulation)
- Demonstrated integration with functional neuroimaging platforms (fMRI, PET, MEG)
- Proven interoperability with common neuroscience software (e.g., SPM, FSL, EEGLAB)
Transaction and Development Safeguards
For custom development or collaborative research, establish clear intellectual property (IP) agreements and milestone-based funding protocols. Utilize escrow arrangements for software source code delivery and require audit rights for algorithmic transparency. Pilot testing is critical—conduct in-lab benchmarking against known neural response patterns before full deployment.
What Are the Best Suppliers for Limbic Cortex Function–Related Research Tools?
No direct suppliers of "limbic cortex function" exist, and the provided supplier data contains no entries relevant to neurotechnology providers. Therefore, no comparative supplier table can be generated based on available input.
In the absence of structured supplier data, procurement professionals are advised to consult independently verified databases such as:
- NIH RePORTER (U.S. National Institutes of Health-funded research)
- European Commission CORDIS database
- Global Medical Device Nomenclature (GMDN) codes for neurodiagnostic equipment
Reputable manufacturers in adjacent domains include firms producing high-density EEG arrays, intracranial recording systems, and real-time fMRI neurofeedback platforms. Selection should be guided by clinical use case, signal fidelity, and post-sale technical support availability.
FAQs
How to verify a supplier's capability in studying limbic cortex function?
Assess technical documentation showing validated measurements of limbic system activity, including specificity for regions like the hippocampus, amygdala, and anterior cingulate cortex. Confirm partnerships with accredited neuroscience laboratories or hospitals conducting functional brain mapping.
What is the typical lead time for neuroscience research equipment?
Standard neuroimaging accessories (e.g., EEG caps, electrode sets): 4–8 weeks. Custom implants or patient-specific neuromodulation devices: 12–24 weeks, depending on regulatory pathway and sterilization requirements.
Can these technologies be shipped internationally?
Yes, but international shipments of medical-grade neurotechnology are subject to export controls (e.g., U.S. ITAR/EAR regulations if software includes AI-based neural decoding), import licensing, and customs classification under HS Code 9018.19 (electroencephalographs) or 9021.50 (implantable neurostimulators).
Do suppliers offer free samples or trial systems?
Samples are rare due to sterility, calibration, and regulatory constraints. However, some vendors provide demo units or cloud-based software trials for algorithm evaluation. On-site demonstrations may be arranged under non-disclosure agreements.
How to initiate customization for specific limbic system research?
Submit detailed specifications including target brain region, spatial/temporal resolution needs, subject population (human/animal), and compatibility requirements (e.g., MRI-safe materials, wireless telemetry). Leading suppliers respond with feasibility assessments within 10–15 business days, followed by prototype development timelines of 8–16 weeks.