Converting atoms to grams may seem like a task reserved for advanced chemistry labs, but it’s a fundamental skill used in everything from pharmaceutical research to environmental science. Whether you're a student tackling stoichiometry or a professional verifying material purity, understanding how to convert the number of atoms into measurable mass is essential. This guide breaks down the process into clear, manageable steps, using foundational concepts like Avogadro’s number and molar mass. By the end, you’ll be able to perform these conversions with confidence and precision.
Understanding the Core Concepts
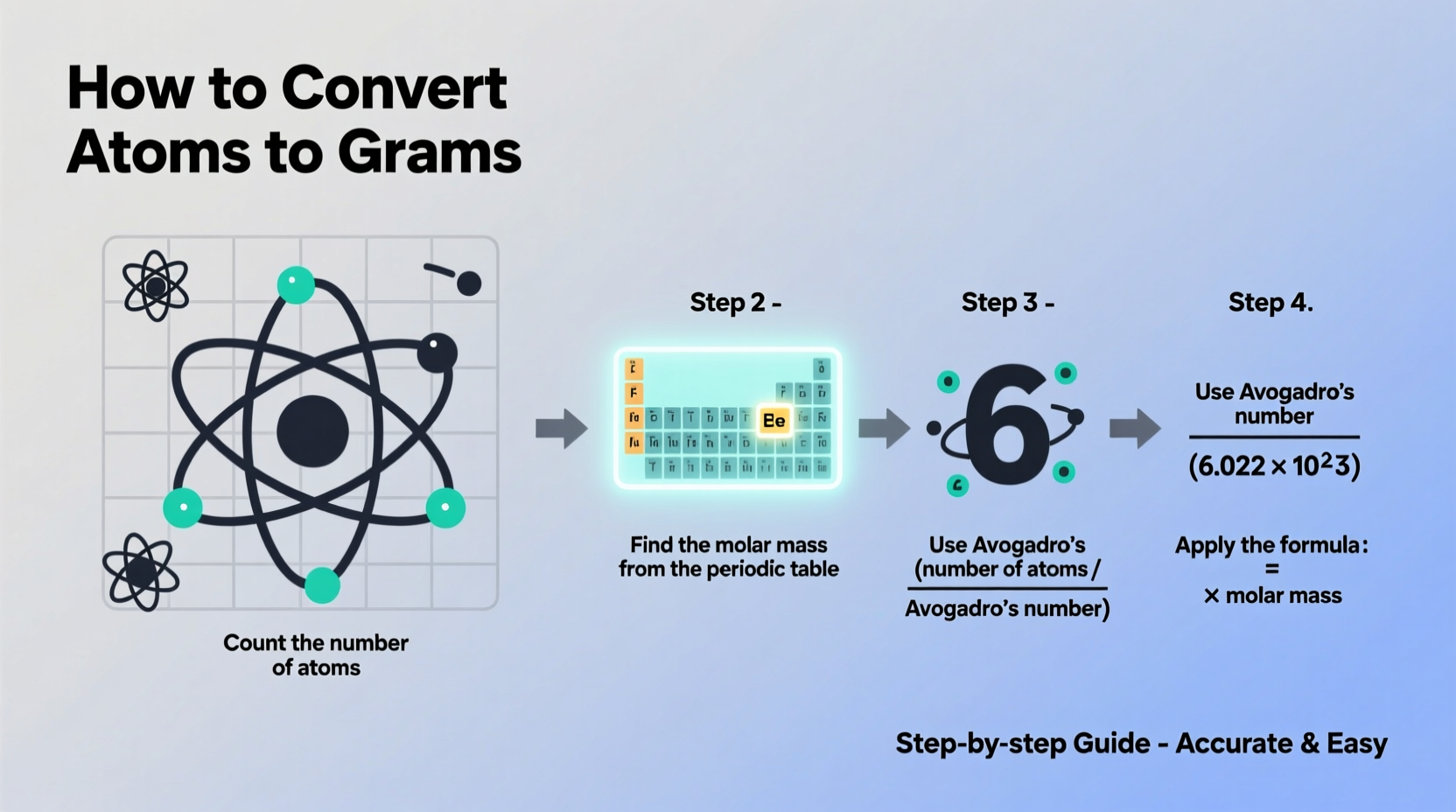
Before diving into calculations, it’s crucial to understand two key scientific constants: Avogadro’s number and molar mass.
Avogadro’s number (6.022 × 10²³) represents the number of atoms, molecules, or particles in one mole of a substance. It acts as a bridge between the microscopic world of atoms and the macroscopic world we measure in grams.
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). You can find this value on the periodic table as the atomic weight of an element. For compounds, sum the atomic weights of all constituent atoms.
“Mastering unit conversions involving atoms builds a strong foundation for all quantitative work in chemistry.” — Dr. Alan Reyes, Physical Chemistry Instructor, MIT
Step-by-Step Conversion Process
Converting atoms to grams involves three logical steps. Follow this sequence carefully to ensure accuracy:
- Determine the number of atoms you are working with.
- Convert the number of atoms to moles using Avogadro’s number.
- Convert moles to grams using the molar mass of the element or compound.
Step 1: Identify the Number of Atoms
This value is usually given in the problem. For example, “How many grams are in 3.5 × 10²² atoms of carbon?” Here, the number of atoms is 3.5 × 10²².
Step 2: Convert Atoms to Moles
Use Avogadro’s number as a conversion factor:
Moles = (Number of atoms) ÷ (6.022 × 10²³ atoms/mol)
For our example: Moles of C = (3.5 × 10²²) / (6.022 × 10²³) ≈ 0.0581 mol
Step 3: Convert Moles to Grams
Multiply the number of moles by the molar mass of the element. Carbon has a molar mass of 12.01 g/mol.
Grams = Moles × Molar Mass = 0.0581 mol × 12.01 g/mol ≈ 0.698 grams
Therefore, 3.5 × 10²² atoms of carbon weigh approximately 0.698 grams.
Handling Compounds: A Real Example
The same principles apply when dealing with molecules. Let’s say you want to convert 2.4 × 10²⁴ molecules of water (H₂O) to grams.
Step 1: Count the Molecules
You’re given 2.4 × 10²⁴ molecules of H₂O.
Step 2: Convert Molecules to Moles
Moles of H₂O = (2.4 × 10²⁴) / (6.022 × 10²³) ≈ 3.985 mol
Step 3: Determine Molar Mass of Water
H₂O = (2 × 1.008 g/mol for hydrogen) + (1 × 16.00 g/mol for oxygen) = 18.016 g/mol
Step 4: Convert Moles to Grams
Mass = 3.985 mol × 18.016 g/mol ≈ 71.8 grams
So, 2.4 × 10²⁴ molecules of water have a mass of about 71.8 grams.
“Students who visualize the mole concept as a ‘chemist’s dozen’ often grasp conversions faster.” — Prof. Lila Nguyen, STEM Education Researcher
Common Pitfalls and How to Avoid Them
Even experienced learners make mistakes in unit conversions. Here’s a checklist to help you stay accurate:
- Always verify whether you're working with atoms, ions, or molecules—this affects molar mass calculation.
- Use scientific notation consistently to avoid decimal errors.
- Ensure units cancel correctly during dimensional analysis.
- Round only at the final step to preserve precision.
- Double-check molar masses, especially for polyatomic ions or hydrates.
| Do | Don’t |
|---|---|
| Use parentheses in calculators when dividing by Avogadro’s number | Assume all elements have whole-number molar masses |
| Cross-check molar mass with the periodic table | Forget that diatomic elements (like O₂) have doubled molar mass |
| Write out each conversion step clearly | Skip units during calculations |
| Estimate results mentally before calculating (e.g., 6 × 10²³ atoms ≈ 1 mole) | Trust calculator output without reasonableness checks |
Practical Applications in Science and Industry
Accurate atom-to-gram conversions are vital beyond the classroom. In pharmaceutical development, scientists must know exactly how many drug molecules are present in a dose. Environmental chemists use these calculations to quantify pollutant concentrations at the molecular level.
Consider a lab technician analyzing a sample of gold nanoparticles. If they count 1.2 × 10²¹ gold atoms using electron microscopy, they need to determine the mass for dosage control.
Mini Case Study: Gold Nanoparticle Dosage
A biomedical researcher isolates a batch containing 1.2 × 10²¹ atoms of gold (Au). What is its mass?
Step 1: Convert atoms to moles Moles = 1.2 × 10²¹ / 6.022 × 10²³ ≈ 0.001993 mol
Step 2: Use molar mass of Au = 196.97 g/mol Mass = 0.001993 mol × 196.97 g/mol ≈ 0.392 grams
The sample weighs nearly 392 milligrams—critical information for controlled medical applications.
Frequently Asked Questions
Can I convert grams to atoms using the same method?
Yes. Reverse the process: divide grams by molar mass to get moles, then multiply by Avogadro’s number to get atoms. For example, 1 gram of hydrogen (1.008 g/mol) contains about 5.97 × 10²³ atoms.
What if the substance is a compound like NaCl?
Treat it the same way, but use the total molar mass of the compound. For NaCl: 22.99 + 35.45 = 58.44 g/mol. Then proceed with moles and Avogadro’s number as usual.
Is Avogadro’s number the same for all substances?
Absolutely. One mole of any substance—whether iron, oxygen gas, or DNA fragments—contains exactly 6.022 × 10²³ elementary entities. The difference lies in their molar mass, not the number of particles per mole.
Conclusion: Master the Mole, Master the Measurement
Converting atoms to grams is more than a textbook exercise—it’s a gateway to precise scientific reasoning. By grounding your calculations in Avogadro’s number and molar mass, you gain the ability to move seamlessly between the invisible world of atoms and the tangible world of mass measurements. With practice, these conversions become second nature, empowering you in academic, industrial, and research settings.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?