When standing in a pharmacy aisle or reviewing an insurance formulary, many patients face a simple yet consequential decision: choose the brand-name medication or its generic counterpart. The price difference can be dramatic—sometimes hundreds of dollars—but what about the medical difference? Is the active ingredient in a generic drug truly identical to that in the brand-name version? And if so, why do concerns persist among patients and even some healthcare providers?
The answer lies at the intersection of pharmaceutical science, regulatory oversight, and consumer perception. While the core active ingredient must be chemically identical, the full story includes nuances in formulation, absorption, and patient experience that influence outcomes.
What Defines a Generic Medication?
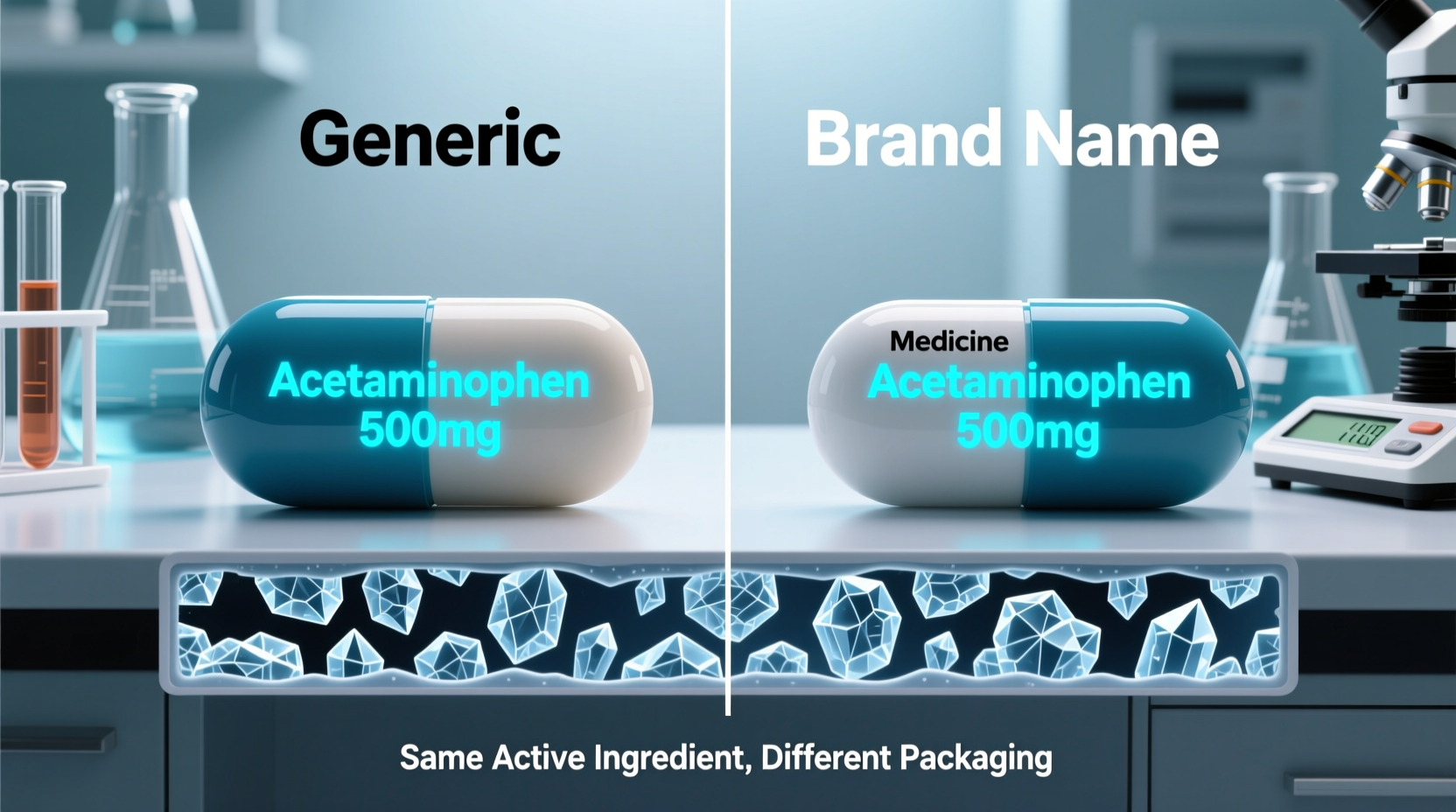
A generic drug is a copy of a brand-name medication that has been approved for use after the original drug’s patent expires. According to the U.S. Food and Drug Administration (FDA), a generic must contain the same:
- Active ingredient
- Strength
- Dosage form (e.g., tablet, liquid, injection)
- Route of administration (e.g., oral, topical)
- Quality, safety, and performance characteristics
The key requirement is bioequivalence—the generic must deliver the same amount of active ingredient into a patient’s bloodstream in the same timeframe as the brand-name drug. This ensures therapeutic equivalence, meaning it should work the same way in treating the condition.
Are Active Ingredients Truly Identical?
Yes—by law and by scientific standard, the active pharmaceutical ingredient (API) in a generic drug must be identical in molecular structure to that in the brand-name version. For example, the API in generic ibuprofen is chemically indistinguishable from that in Advil®. It is synthesized to meet the same pharmacopeial standards and undergoes rigorous testing.
However, inactive ingredients—such as fillers, binders, dyes, and preservatives—can differ. These excipients do not affect the drug’s primary action but may influence tolerability. Some patients report gastrointestinal discomfort, allergic reactions, or changes in efficacy when switching brands due to these non-active components.
The FDA requires generics to have the same strength, purity, and potency as their brand-name counterparts. Manufacturers must prove consistency across batches and demonstrate stability under various conditions. Any variation in the API beyond tightly defined limits would disqualify the product from approval.
“Generic drugs are not ‘copycats’ in the derogatory sense. They are scientifically validated equivalents.” — Dr. Janet Woodcock, Former Director, FDA Center for Drug Evaluation and Research
Regulatory Oversight: How Generics Are Approved
In the United States, the Hatch-Waxman Act of 1984 established the modern framework for generic drug approval. Instead of repeating costly clinical trials, generic manufacturers submit an Abbreviated New Drug Application (ANDA). This process relies on proving bioequivalence through pharmacokinetic studies—typically in healthy volunteers—who receive both the brand-name and generic versions in a crossover design.
Bioequivalence is assessed using two main metrics:
- Cmax: Peak concentration of the drug in the blood
- AUC: Area under the curve, representing total drug exposure over time
The FDA allows a 90% confidence interval for the ratio of generic to brand within 80% to 125%. This range accounts for normal biological variability while ensuring clinical interchangeability.
Despite this margin, real-world evidence overwhelmingly supports the effectiveness of generics. A 2017 study published in the Journal of the American Medical Association analyzed over 3 million patients and found no significant differences in effectiveness or safety between brand-name and generic cardiovascular drugs.
Step-by-Step: How a Generic Drug Reaches the Market
- Patent Expiration: The original drug’s patent protection ends (typically 20 years from filing).
- ANDA Submission: A generic manufacturer applies to the FDA with data on formulation, manufacturing, and bioequivalence.
- Facility Inspection: The FDA inspects production sites for compliance with Good Manufacturing Practices (GMP).
- Bioequivalence Testing: Human studies compare absorption rates between generic and brand.
- Approval & Launch: Once approved, the generic enters the market, often at 30–80% lower cost.
Perception vs. Reality: Why Doubts Persist
Despite strong scientific backing, skepticism about generics remains. Surveys show that up to 40% of Americans believe brand-name drugs are more effective, even when informed they contain the same active ingredient.
Several factors contribute to this perception gap:
- Marketing Influence: Brand-name companies spend billions annually reinforcing trust in their products.
- Physical Differences: Color, shape, or size variations in pills can make patients feel the medication is different.
- Anecdotal Reports: Individual experiences of side effects or reduced efficacy—often due to excipients or placebo effect—are amplified online.
- Insurance Mandates: Patients forced to switch may blame the drug rather than adjust to physiological fluctuations.
One notable case involves levothyroxine, a thyroid hormone replacement. Though generics are FDA-approved, some patients report symptoms returning after switching brands. This has led to recommendations that patients stay on a consistent formulation once stabilized.
Mini Case Study: Managing Hypertension with Lisinopril
Maria, a 62-year-old with stage 1 hypertension, was prescribed brand-name Zestril® for years. When her insurance changed, she was switched to generic lisinopril without consultation. Within weeks, she reported dizziness and fatigue. Her doctor initially suspected poor adherence but later discovered her pharmacy had alternated between three different generic manufacturers monthly due to supply contracts.
After switching to a single-source generic supplier and monitoring blood pressure closely, Maria’s symptoms resolved. The issue wasn’t the generic drug itself, but inconsistency in inactive ingredients affecting absorption. This highlights the importance of formulation stability—even within approved generics.
Do’s and Don’ts: Navigating the Generic Decision
| Do | Don't |
|---|---|
| Ask your pharmacist which manufacturer produced your generic | Assume all generics of the same drug are interchangeable overnight |
| Keep track of how you feel after switching medications | Stop taking medication because of appearance changes |
| Use manufacturer loyalty programs or discount cards if cost is still high | Pay full price for a brand-name drug without checking for available generics |
| Discuss concerns with your doctor—especially for seizure, heart, or mental health medications | Rely solely on anecdotal stories from forums or social media |
When to Be Cautious: Narrow Therapeutic Index Drugs
While most generics perform equivalently, special caution is advised for drugs with a narrow therapeutic index (NTI)—those where small differences in blood concentration can lead to toxicity or treatment failure. Examples include:
- Warfarin (blood thinner)
- Phenytoin (anti-seizure)
- Lithium (mood stabilizer)
- Digoxin (heart medication)
For these medications, some states restrict automatic substitution at the pharmacy level unless the prescriber indicates “dispense as written” or “substitution allowed.” The American College of Clinical Pharmacy recommends that patients on NTI drugs remain on a consistent product whenever possible.
Frequently Asked Questions
Can generic drugs look different from brand-name ones?
Yes. Trademark laws prevent generics from appearing identical to brand-name drugs. Differences in color, shape, or imprint are common and do not affect efficacy. However, these changes can confuse patients, so it helps to review new pills with a pharmacist.
Why are generic drugs cheaper?
Generic manufacturers do not bear the costs of initial research, development, or marketing. They enter the market after patents expire and compete on price. This competition drives down costs for consumers and insurers alike. On average, generics cost 80–85% less than brand-name versions.
Are generic drugs made in less-regulated countries safe?
Many generic APIs are manufactured overseas—particularly in India and China—but they must meet the same FDA standards as U.S.-based facilities. The FDA conducts inspections of foreign plants and can issue import alerts for non-compliant sites. While challenges exist, there is no evidence that foreign-made generics are systematically less safe.
Expert Insight: What Doctors Wish Patients Knew
“We spend a lot of time convincing patients that generics are just as good. The data is clear—they work the same. But we also need to listen when someone says they feel differently on a new version. Sometimes it’s psychological, sometimes it’s the fillers. Either way, it deserves attention.” — Dr. Rajiv Patel, Internal Medicine Specialist
Healthcare providers emphasize shared decision-making. While generics are appropriate for the vast majority of patients, individual responses matter. Open communication between patient, pharmacist, and physician ensures optimal outcomes.
Conclusion: Making an Informed Choice
The active ingredient in generic medications is, by regulatory and chemical definition, identical to that in brand-name drugs. Rigorous testing ensures bioequivalence, and decades of clinical use support their safety and effectiveness. Cost savings are substantial, making essential treatments accessible to millions.
Yet, individual experiences vary. Inactive ingredients, manufacturing consistency, and patient expectations all play roles in how a medication is perceived and tolerated. Being informed—knowing when to question a switch, understanding the role of excipients, and maintaining dialogue with healthcare providers—is key to making confident choices.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?