Potassium (K⁺) leak channels are fundamental components of cellular physiology, quietly shaping the electrical behavior of nearly every excitable and non-excitable cell in the body. Unlike voltage-gated or ligand-gated ion channels that open and close in response to specific stimuli, K⁺ leak channels operate continuously, allowing potassium ions to passively diffuse out of the cell. This steady outward movement is a primary determinant of the resting membrane potential—the baseline electrical charge difference across the cell membrane when the cell is not actively signaling.
Understanding which choice best characterizes K⁺ leak channels requires more than memorizing textbook definitions; it demands insight into their biophysical properties, physiological roles, and how they differ from other types of ion channels. Whether you're studying for an exam, researching neuronal excitability, or exploring cardiac electrophysiology, grasping the nature of these channels is essential.
The Biophysics of K⁺ Leak Channels
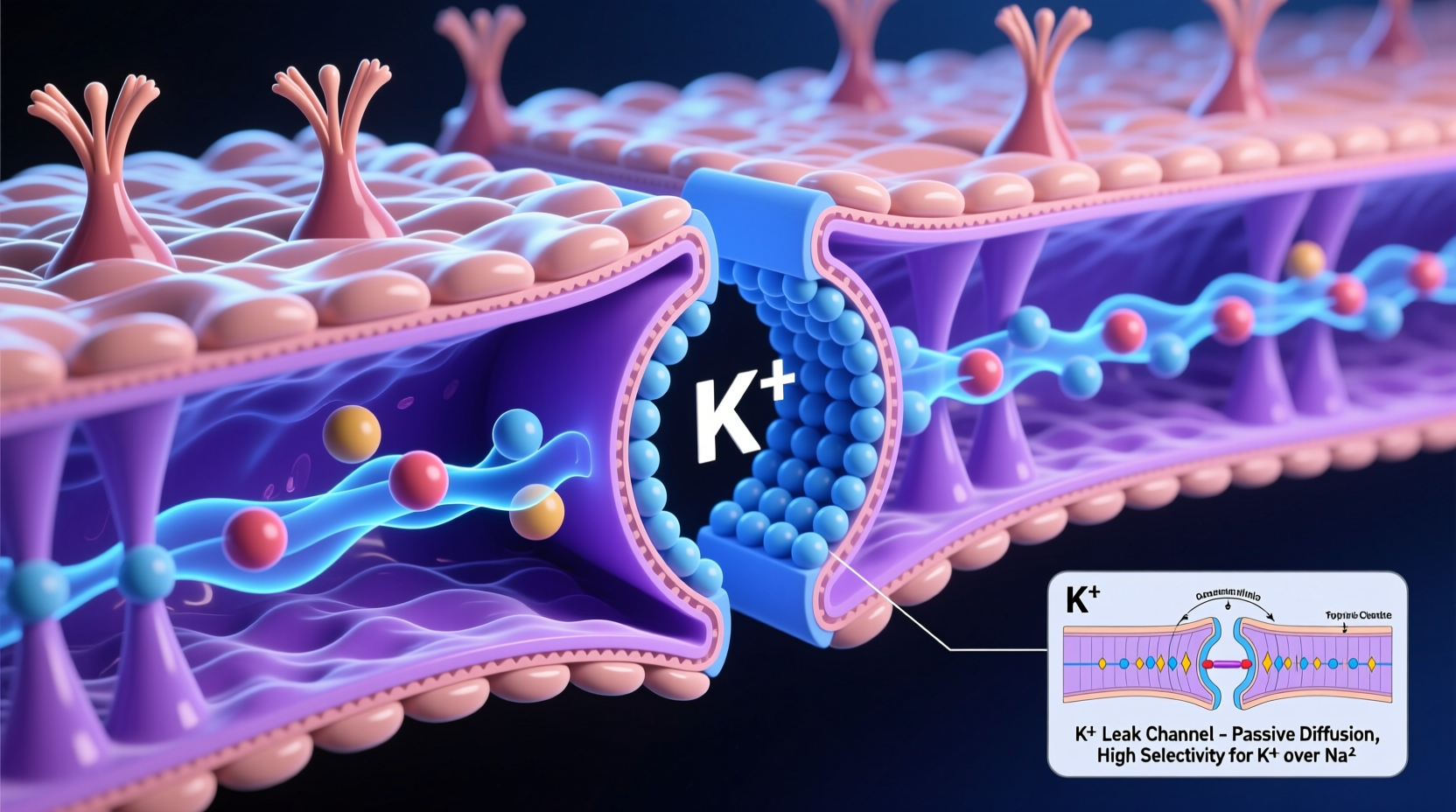
K⁺ leak channels are transmembrane proteins embedded in the lipid bilayer. Their defining feature is their \"leakiness\"—they remain open under resting conditions, permitting K⁺ ions to move down their electrochemical gradient. Because intracellular K⁺ concentration is much higher than extracellular concentration, potassium naturally diffuses outward. This efflux of positive charge makes the inside of the cell more negative relative to the outside, directly contributing to the resting membrane potential of approximately -70 mV in most mammalian cells.
These channels exhibit high selectivity for potassium over other cations like sodium (Na⁺), thanks to a conserved amino acid sequence known as the selectivity filter. The structure of this filter mimics the hydration shell of K⁺, allowing the ion to shed its water molecules and pass through with minimal energy cost—a phenomenon explained by the pioneering work of Roderick MacKinnon, who won the Nobel Prize in Chemistry in 2003 for his studies on ion channel structure.
How K⁺ Leak Channels Differ from Other Ion Channels
Ion channels are broadly classified based on their gating mechanisms: voltage-gated, ligand-gated, mechanically-gated, and leak channels. While all facilitate ion movement, only leak channels lack a regulatory switch. They are always open, making them ideal for maintaining stable background conductance.
| Channel Type | Gating Mechanism | Role | Example |
|---|---|---|---|
| K⁺ Leak Channels | Constitutively open | Maintain resting potential | TASK, TREK families |
| Voltage-Gated K⁺ Channels | Open with depolarization | Repolarize action potentials | Kv1.1, Kv7.1 |
| Ligand-Gated K⁺ Channels | Activated by neurotransmitters | Modulate synaptic inhibition | GIRK channels |
| Mechanosensitive K⁺ Channels | Respond to membrane stretch | Regulate volume and pressure | TREK-1 |
This distinction is crucial when evaluating multiple-choice questions about ion channel function. A common misconception is that all potassium channels are involved in repolarizing action potentials. In reality, K⁺ leak channels primarily stabilize the resting state rather than participate in rapid signaling events.
“Leak channels set the stage upon which all other electrical activity occurs. Without them, neurons couldn’t maintain a baseline from which to fire.” — Dr. Laura Chen, Neurophysiologist, Stanford University
Physiological Significance Across Tissues
The importance of K⁺ leak channels extends beyond basic cellular biology. In neurons, they help regulate excitability. Increased K⁺ leak current hyperpolarizes the membrane, making it harder for the cell to reach the threshold for firing an action potential. This serves as a natural brake on excessive neural activity.
In the heart, certain K⁺ leak channels contribute to stabilizing the resting potential of pacemaker cells. Although the sinoatrial node relies heavily on funny currents (If) for rhythmicity, background K⁺ conductance helps fine-tune automaticity and prevents arrhythmias.
Even in non-excitable cells like glia or epithelial cells, K⁺ leak channels play critical roles in potassium spatial buffering and transepithelial transport. For instance, in the kidney, some two-pore domain K⁺ (K2P) leak channels assist in K⁺ secretion, influencing electrolyte balance and blood pressure regulation.
Mini Case Study: Anesthesia and TREK-1 Channels
A compelling example of K⁺ leak channel relevance comes from research on general anesthetics. Studies have shown that volatile anesthetics such as halothane and isoflurane activate TREK-1, a member of the K2P family. By enhancing K⁺ efflux, these drugs hyperpolarize neurons, reducing overall brain activity and contributing to loss of consciousness.
This discovery has shifted understanding of how anesthetics work—not solely through GABAergic enhancement, but also via direct modulation of background potassium currents. It underscores that K⁺ leak channels are not passive bystanders but dynamic regulators capable of being pharmacologically targeted.
Common Misconceptions and Clarifications
When asked to identify the best characterization of K⁺ leak channels, students often face distractors emphasizing activation mechanisms or involvement in action potentials. Let’s clarify:
- Myth: K⁺ leak channels open in response to depolarization.
Reality: They are voltage-independent and open regardless of membrane potential. - Myth: They are responsible for repolarizing the cell after an action potential.
Reality: That role belongs to voltage-gated K⁺ channels (e.g., delayed rectifiers). - Myth: All potassium channels are the same.
Reality: Over 70 distinct K⁺ channel genes exist in humans, serving diverse functions.
Checklist: Identifying True K⁺ Leak Channel Characteristics
To determine which choice best characterizes K⁺ leak channels, use this checklist:
- Are they open at rest? → Yes
- Do they require a stimulus to open? → No
- Do they contribute significantly to resting membrane potential? → Yes
- Are they highly selective for K⁺? → Yes
- Do they rapidly inactivate? → No (they show little to no inactivation)
- Are they part of the K2P family in many cases? → Yes
If a multiple-choice option aligns with these traits—especially constitutive activity and role in resting potential—it is likely the correct answer.
Frequently Asked Questions
What is the main function of K⁺ leak channels?
Their primary function is to establish and stabilize the resting membrane potential by allowing a steady outward flow of potassium ions, which makes the interior of the cell more negative.
Are K⁺ leak channels the same as inward-rectifier K⁺ channels?
No. While both contribute to resting potential, inward-rectifier (Kir) channels allow more inward current at negative potentials and are regulated by intracellular factors like ATP or G-proteins. K⁺ leak channels, particularly K2P types, are voltage-insensitive and always conduct outward K⁺ current.
Can K⁺ leak channels be blocked?
Yes, though they are less sensitive to classic K⁺ blockers like tetraethylammonium (TEA). Some K2P channels can be inhibited by extracellular acidosis, mechanical stretch, or specific toxins. Pharmacological targeting remains an active area of research.
Conclusion: Why Accurate Characterization Matters
Understanding which choice best characterizes K⁺ leak channels isn't just academic—it's foundational for interpreting everything from neuropharmacology to cardiac rhythms. These channels are the silent architects of cellular stability, enabling cells to maintain readiness for action without drifting into uncontrolled excitation.
When evaluating options, focus on key descriptors: “always open,” “voltage-independent,” “maintains resting potential,” and “passive diffusion of K⁺.” Avoid choices implying regulation by voltage, ligands, or involvement in spike repolarization.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?