When you pour water into a glass beaker or graduated cylinder, you may have noticed that the surface of the liquid doesn’t remain perfectly flat. Instead, it curves near the edges—forming what scientists call a meniscus. This subtle curvature is more than just a visual quirk; it’s a direct result of the fundamental forces governing how liquids interact with their environment. Understanding why a meniscus forms provides insight into surface tension, adhesion, cohesion, and the behavior of fluids at microscopic scales.
The presence of a meniscus affects everything from laboratory measurements to industrial fluid dynamics. Whether you're reading volume in a chemistry lab or designing microfluidic devices, recognizing the origin and implications of this phenomenon is essential. Let’s explore the physics behind meniscus formation and its real-world consequences.
The Science Behind Surface Curvature
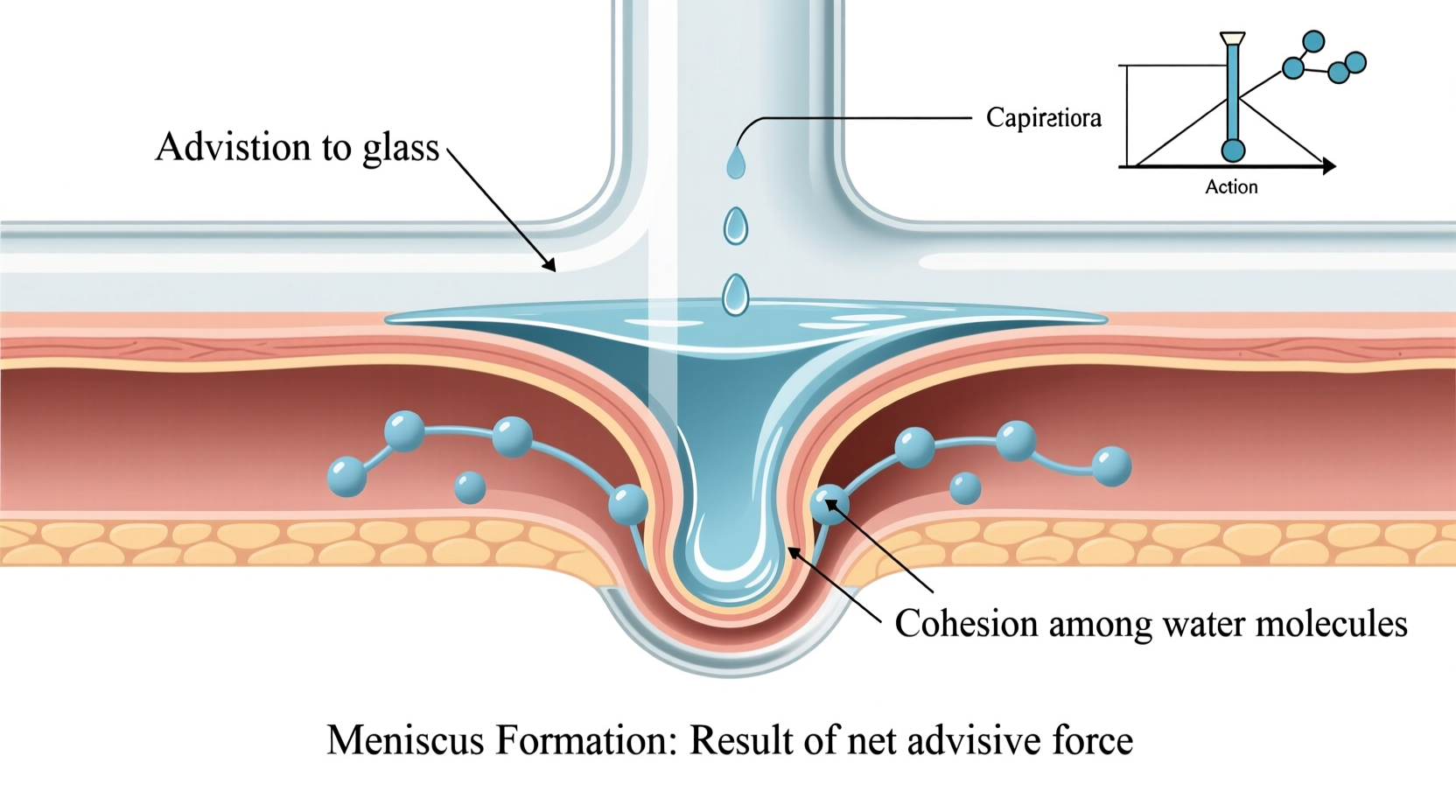
A meniscus is the curved surface of a liquid at the interface between the liquid and its container. It arises due to two competing intermolecular forces: adhesion and cohesion.
- Cohesion: The attraction between molecules of the same substance. In water, hydrogen bonding causes strong cohesive forces, pulling molecules inward and minimizing surface area.
- Adhesion: The attraction between unlike substances—such as water molecules and the glass wall of a container. When adhesive forces exceed cohesive ones, the liquid climbs slightly up the surface.
In polar liquids like water, adhesion to glass (which has charged silanol groups on its surface) is stronger than cohesion within the liquid. As a result, water molecules are pulled upward along the container walls, creating a concave meniscus—a curve that dips downward in the center and rises at the edges.
Conversely, nonpolar liquids such as mercury exhibit stronger cohesive forces than adhesive ones. Mercury molecules are more attracted to each other than to glass, causing them to pull away from the container walls. This results in a convex meniscus—an upward bulge in the center.
Role of Surface Tension and Contact Angle
Surface tension plays a crucial role in shaping the meniscus. It acts like an elastic skin on the surface of a liquid, resisting external forces. High surface tension enhances both cohesion and the tendency to form a distinct meniscus.
The degree to which a liquid wets a surface is quantified by the contact angle—the angle formed where the liquid meets the solid boundary. This angle determines whether the meniscus will be concave or convex:
| Contact Angle | Meniscus Type | Liquid Example |
|---|---|---|
| < 90° | Concave | Water in glass |
| > 90° | Convex | Mercury in glass |
| ≈ 0° | Highly concave | Alcohol in clean glass |
A contact angle less than 90 degrees indicates favorable wetting, meaning adhesion dominates. Angles greater than 90 degrees suggest poor wetting, where cohesion wins out. The precise shape of the meniscus can even be modeled mathematically using Young-Laplace equations, which balance pressure differences across curved interfaces with surface tension and radius of curvature.
Capillary Action: When Meniscus Drives Flow
The formation of a meniscus isn't just static—it can initiate motion. Capillary action occurs when the meniscus pulls liquid up narrow tubes against gravity. This process powers many natural and engineered systems.
For example, in plants, water travels from roots to leaves through xylem vessels via capillarity enhanced by transpiration. Similarly, paper towels absorb spills because cellulose fibers create tiny channels where adhesive forces draw liquid upward.
The height to which a liquid rises in a capillary tube depends on several factors:
- Diameter of the tube – narrower tubes produce higher rise
- Surface tension of the liquid – higher tension increases lift
- Density of the liquid – denser liquids rise less
- Contact angle – lower angles enhance climbing ability
This principle is exploited in medical diagnostics, inkjet printing, and soil moisture transport. Without the meniscus-induced imbalance of forces, these processes would not function efficiently.
Mini Case Study: Measuring Errors in Chemistry Labs
In high school and university chemistry labs, students often use graduated cylinders to measure liquid volumes. One common mistake is misreading the meniscus, especially under poor lighting or at incorrect angles.
A student once recorded a 25.0 mL volume of water but viewed the meniscus from above instead of at eye level. Due to parallax error and failure to align with the bottom of the curve, the actual volume was closer to 24.3 mL—a nearly 3% error. In titration experiments requiring precision, such discrepancies can invalidate results.
After receiving feedback, the student adopted a checklist for proper technique, significantly improving accuracy. This highlights how understanding the meniscus isn’t merely theoretical—it directly impacts experimental reliability.
Expert Insight on Fluid Interfaces
“Interfacial phenomena like the meniscus reveal how molecular-scale interactions govern macroscopic behavior. Misunderstanding them leads to errors in research, engineering, and even everyday applications.” — Dr. Alan Reyes, Fluid Dynamics Researcher, MIT
Dr. Reyes emphasizes that while the meniscus appears simple, it serves as a gateway to deeper concepts in soft matter physics and materials science. From coating technologies to lab-on-a-chip devices, controlling meniscus shape enables innovation.
Practical Tips for Accurate Observations
To ensure correct interpretation and usage of menisci, particularly in scientific contexts, follow these best practices:
- Always position your eyes level with the meniscus to avoid parallax distortion.
- Clean glassware thoroughly—grease or residue alters adhesion and distorts the curve.
- Use appropriately sized equipment; large containers reduce curvature visibility.
- Be aware of temperature effects—surface tension changes with heat, altering meniscus shape.
- For digital sensors or automated systems, calibrate based on expected meniscus profile.
Common Questions About Meniscus Behavior
Why do some liquids form upward-curving menisci?
Liquids like mercury form convex menisci because their internal cohesive forces are much stronger than their adhesion to container walls. The liquid minimizes contact with the solid surface, pulling inward and creating a dome-like shape.
Does the material of the container affect the meniscus?
Yes. Glass promotes a concave meniscus in water due to strong adhesion. However, if the same water is placed in a hydrophobic plastic container (like polyethylene), adhesion weakens, resulting in a flatter or even slightly convex meniscus.
Is the meniscus always present?
Technically, yes—even in large containers, a meniscus exists near the edges. However, in wide vessels, its effect on the overall surface is negligible. Only in narrow tubes or precise measuring instruments does it become significant enough to require attention.
Step-by-Step Guide to Reading a Meniscus Correctly
Accurate volume readings depend on proper meniscus observation. Follow this sequence:
- Place the container on a flat, stable surface. Avoid holding it in hand, which introduces instability.
- Ensure adequate lighting. Shadows can obscure the curve.
- Position yourself so your eyes are exactly horizontal with the liquid level. Bend down or adjust the bench height if needed.
- Identify the type of meniscus. For water and most aqueous solutions, locate the lowest point of the curve (concave). For mercury, read the highest point (convex).
- Align the calibration mark with the appropriate part of the meniscus. Use a contrasting background if necessary.
- Record the value immediately to prevent evaporation or disturbance from affecting the reading.
Conclusion: Embracing the Details of Liquid Behavior
The formation of a meniscus is a small yet profound demonstration of how molecular interactions manifest in visible ways. Far from being a mere curiosity, it underpins critical processes in nature, industry, and scientific inquiry. By understanding the balance between adhesion, cohesion, and surface tension, we gain better control over fluid handling and measurement.
Whether you're a student learning to pipette, a researcher designing microchannels, or simply someone fascinated by everyday physics, paying attention to the meniscus enriches your grasp of the physical world. Take time to observe it closely—the curve holds more meaning than meets the eye.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?