When a substance is heated, its volume often increases—a phenomenon observed daily in everything from rising hot air balloons to expanding railway tracks. This behavior is not random but follows fundamental principles of physics rooted in molecular motion and energy transfer. Understanding why volume increases with temperature requires exploring how particles behave when they gain thermal energy. Whether dealing with gases, liquids, or solids, the relationship between heat and expansion reveals consistent patterns governed by scientific laws.
The Molecular Basis of Thermal Expansion
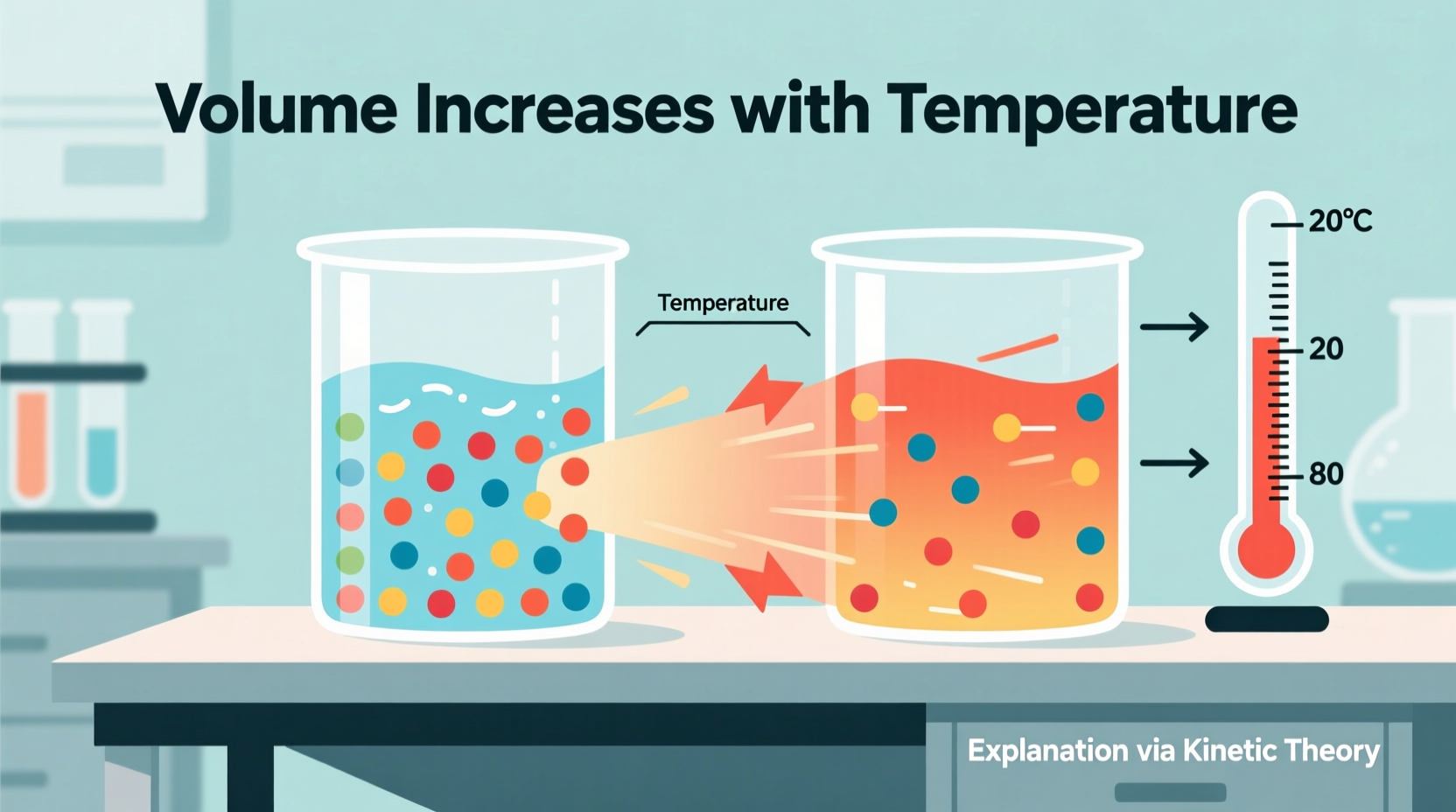
At the atomic and molecular level, all matter consists of particles in constant motion. In solids, these particles vibrate around fixed positions; in liquids, they slide past one another; and in gases, they move freely at high speeds. Temperature is a measure of the average kinetic energy—essentially, the energy of motion—of these particles.
When heat is applied, energy is transferred to the particles, increasing their kinetic energy. As they move more vigorously, they collide more frequently and push each other farther apart. This increased separation leads to an expansion in volume. The extent of this expansion depends on the state of matter:
- Gases: Exhibit the most noticeable expansion because molecules are far apart and interact weakly. Even small increases in energy cause large changes in volume.
- Liquids: Expand less than gases but more than solids due to closer particle proximity and stronger intermolecular forces.
- Solids: Show minimal expansion, as particles are tightly bound, but still expand slightly when heated.
This principle explains why a balloon inflates when warmed or why mercury rises in a thermometer. The added thermal energy forces particles to occupy more space.
Charles’s Law: The Gas Volume-Temperature Relationship
One of the clearest demonstrations of volume change with temperature is found in gases, described quantitatively by Charles’s Law. Formulated in the late 18th century by Jacques Charles, it states:
“At constant pressure, the volume of a given mass of gas is directly proportional to its absolute temperature (measured in Kelvin).”
This means if you double the absolute temperature of a gas, its volume doubles—provided pressure remains unchanged. Mathematically, this is expressed as:
V ∝ T → V/T = k (constant)
For example, consider a sealed piston containing air at room temperature (298 K) occupying 2 liters. If heated to 596 K (twice the original temperature), the volume expands to 4 liters under constant pressure.
This law only holds when using the Kelvin scale, which starts at absolute zero—the theoretical point where all molecular motion ceases. Using Celsius or Fahrenheit would yield incorrect proportions because those scales include negative values unrelated to kinetic energy.
Real-World Application: Hot Air Balloons
A classic illustration of Charles’s Law is the operation of a hot air balloon. When the burner heats the air inside the envelope, the gas molecules gain kinetic energy and spread out. Since the balloon is open at the bottom, some air escapes, reducing density. The warmer, less dense air becomes buoyant relative to the cooler surrounding air, causing the balloon to rise.
“The key to flight in a hot air balloon isn’t just heat—it’s the controlled expansion of air to reduce density.” — Dr. Alan Reeves, Atmospheric Physicist
Thermal Expansion in Solids and Liquids
While gases follow predictable mathematical laws, solids and liquids also expand with temperature, though less dramatically. Engineers must account for this in construction, manufacturing, and design.
In solids, atoms are held in rigid structures by chemical bonds. Heating causes them to vibrate more intensely, effectively increasing the average distance between atoms. Though individual movements are tiny, collectively they result in measurable expansion.
Liquids behave similarly, but their lack of fixed shape allows more freedom of movement. Water, however, exhibits an anomaly: it reaches maximum density at 4°C and expands when cooled further toward freezing, which is why ice floats.
| Material | Linear Expansion Coefficient (per °C) | Effect of 50°C Increase on 10m Sample |
|---|---|---|
| Aluminum | 24 × 10⁻⁶ | +12 mm |
| Steel | 12 × 10⁻⁶ | +6 mm |
| Glass | 9 × 10⁻⁶ | +4.5 mm |
| Concrete | 10 × 10⁻⁶ | +5 mm |
These coefficients determine how much a material will expand per degree of temperature change. Such data is critical in building bridges, laying railroad tracks, and installing pipelines.
Mini Case Study: The Bimetallic Strip in Thermostats
A practical application of differential thermal expansion occurs in household thermostats. These devices use a bimetallic strip, composed of two metals bonded together—typically brass and iron—that expand at different rates when heated.
As temperature rises, brass expands more than iron, causing the strip to bend. This mechanical movement triggers an electrical switch that turns heating or cooling systems on or off. When the room cools, the strip straightens, reversing the process.
This self-regulating mechanism requires no external power and demonstrates how predictable volume changes with temperature can be harnessed for automation. It’s a simple yet effective solution used in furnaces, ovens, and older HVAC controls.
Step-by-Step: Observing Volume Change in a Lab Setting
You can observe the effect of temperature on volume through a basic experiment with common lab equipment:
- Prepare a flask fitted with a rubber stopper and glass tube.
- Partially fill the tube with colored water so it's visible.
- Place the flask in an ice bath and record the initial water level.
- Transfer the flask to warm water (not boiling) and observe the liquid rising in the tube.
- Measure the height change over time as temperature increases.
- Cool the flask again and note whether the liquid returns to its original position.
The rising water indicates that air inside the flask expanded due to increased molecular motion. When cooled, contraction occurs, confirming reversibility. This hands-on demonstration reinforces the direct relationship between temperature and volume in gases.
Frequently Asked Questions
Does volume always increase with temperature?
In most cases, yes—but there are exceptions. Water between 0°C and 4°C contracts when heated, reaching peak density at 4°C. Some engineered materials like zirconium tungstate even exhibit negative thermal expansion, shrinking when heated over certain ranges.
Why do we use Kelvin instead of Celsius in gas laws?
Kelvin is an absolute scale starting at zero kinetic energy. Ratios in gas laws (like V₁/T₁ = V₂/T₂) require absolute temperatures to remain valid. Using Celsius could lead to division by zero or negative volumes, which are physically impossible.
Can pressure affect how volume responds to temperature?
Absolutely. If pressure is held constant, volume increases with temperature. But if volume is constrained (e.g., in a rigid container), then temperature increase results in higher pressure instead—described by Gay-Lussac’s Law.
Actionable Checklist: Managing Thermal Expansion
- ✅ Use expansion joints in long concrete pathways and bridges to allow safe movement.
- ✅ Leave gaps when installing metal rails or piping to accommodate growth.
- ✅ Avoid rapid temperature changes in glassware to prevent cracking from uneven expansion.
- ✅ Calibrate scientific instruments at operating temperatures for accuracy.
- ✅ Design electronic enclosures with ventilation to manage internal heat buildup.
Conclusion: Harnessing Heat-Driven Expansion
The increase in volume with temperature is more than a textbook concept—it’s a force shaping engineering decisions, natural phenomena, and everyday experiences. From the precision of laboratory instruments to the grand scale of infrastructure, understanding thermal expansion enables safer, smarter designs. By recognizing how energy translates into motion and space, we gain deeper insight into the invisible dance of molecules that governs our physical world.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?