Water is one of the most abundant and essential substances on Earth, vital not only for sustaining life but also for enabling countless chemical processes. Its ability to dissolve more substances than any other liquid earns it the title of “universal solvent.” While no solvent is truly universal—some materials like oils and waxes resist dissolution—water’s unmatched versatility in breaking down ionic and polar compounds makes it indispensable in biological, environmental, and industrial contexts.
This distinction isn’t just a scientific curiosity; it has profound implications for how nutrients move through ecosystems, how cells function, and even how pollution spreads. Understanding why water holds this title requires examining its molecular structure, polarity, hydrogen bonding, and real-world applications across natural systems.
The Molecular Basis of Water’s Solvent Power
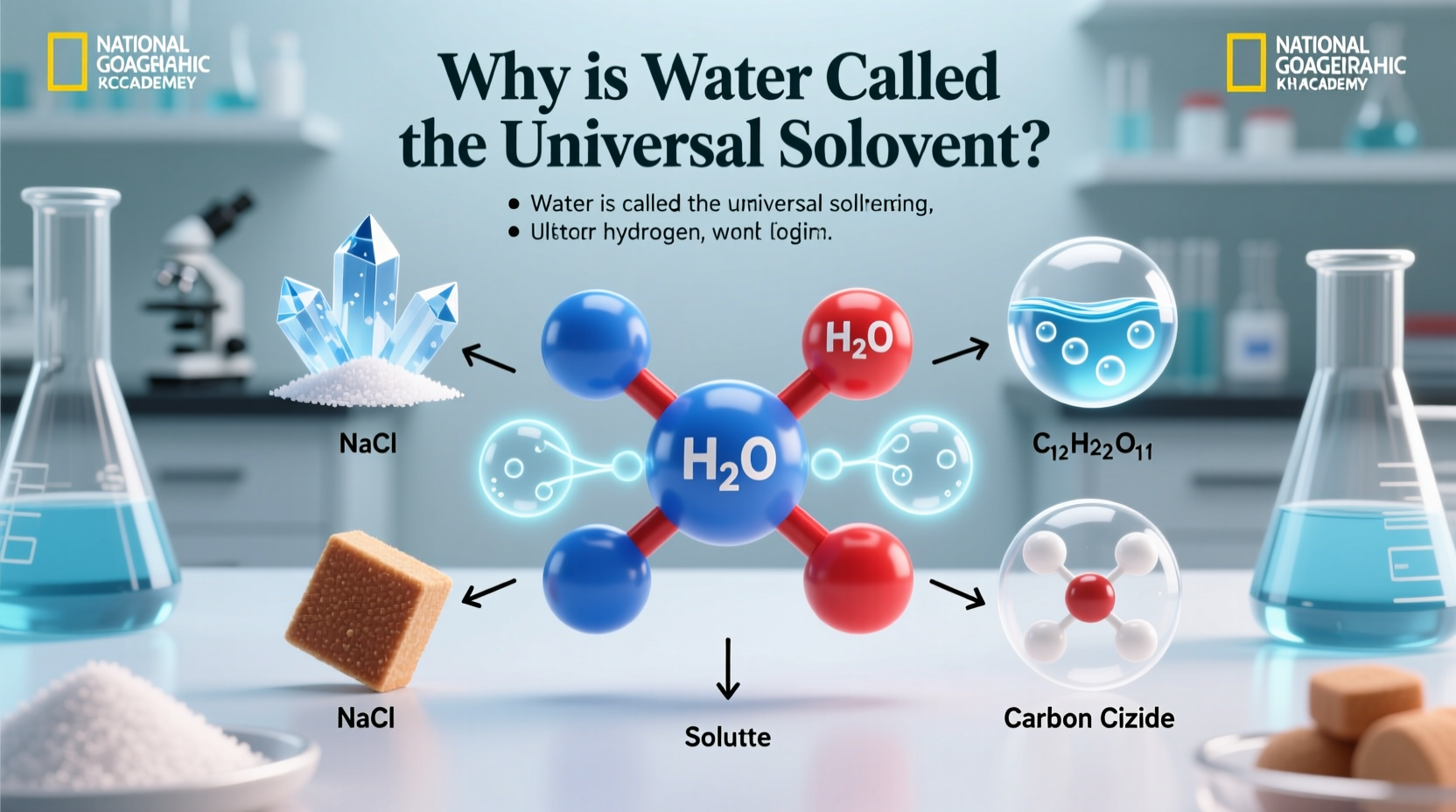
At the heart of water’s effectiveness as a solvent lies its molecular structure. A water molecule (H₂O) consists of two hydrogen atoms covalently bonded to one oxygen atom. This arrangement creates an asymmetrical shape with a bent geometry, resulting in an uneven distribution of electrical charge.
The oxygen atom has a higher electronegativity than hydrogen, meaning it pulls electrons closer to itself. This gives the oxygen end a partial negative charge (δ⁻) and the hydrogen ends a partial positive charge (δ⁺), making water a highly polar molecule. When ionic compounds such as sodium chloride (NaCl) enter water, the positively charged sodium ions are attracted to the oxygen ends of water molecules, while the negatively charged chloride ions are drawn to the hydrogen ends.
This interaction surrounds the ions and pulls them apart from the crystal lattice—a process known as dissociation. Because of this, salts, acids, bases, and many other polar substances readily dissolve in water, allowing them to participate in biochemical reactions.
Hydrogen Bonding and Cohesion
Beyond simple polarity, water molecules form hydrogen bonds with each other and with solutes. Each water molecule can engage in up to four hydrogen bonds—two through its hydrogen atoms and two via lone electron pairs on oxygen. These transient but numerous interactions give water high cohesion, surface tension, and heat capacity.
In solution, hydrogen bonding enhances water’s ability to stabilize dissolved ions and molecules. For example, when glucose dissolves in water, its hydroxyl (-OH) groups form hydrogen bonds with surrounding water molecules, effectively shielding it and preventing re-aggregation. This property allows sugars, amino acids, and other biomolecules to remain dispersed and available for metabolic use.
Moreover, hydrogen bonding contributes to water’s high dielectric constant—the measure of a substance’s ability to reduce electrostatic forces between charged particles. With a dielectric constant of about 80, water significantly weakens the attraction between oppositely charged ions, facilitating their separation and increasing solubility.
“Water’s polarity and hydrogen-bonding network make it uniquely suited to support life by dissolving and mobilizing essential biochemicals.” — Dr. Linda Chen, Biophysical Chemist, MIT
Biological Significance of Water as a Solvent
In living systems, water’s role as a solvent is foundational. Cellular processes—from enzyme catalysis to signal transduction—occur in aqueous environments. Blood plasma, lymph, cytoplasm, and interstitial fluids are all water-based solutions that transport gases, hormones, ions, and nutrients throughout the body.
Consider digestion: hydrochloric acid in the stomach dissolves food particles, while enzymes in the small intestine operate in a watery medium to break down macromolecules into absorbable units. Similarly, in plants, xylem vessels carry mineral ions dissolved in water from roots to leaves, enabling photosynthesis and growth.
Even at the cellular level, the movement of potassium and sodium ions across membranes—critical for nerve impulses—depends on their solubility in intracellular and extracellular fluids. Without water’s dissolving capability, these electrochemical gradients could not be established or maintained.
Real-World Example: Nutrient Uptake in Agriculture
A farmer applying fertilizer to crops relies on water’s solvent properties. Dry granules of potassium nitrate or ammonium phosphate don’t directly feed plants. Instead, rain or irrigation water dissolves these compounds into ions (K⁺, NO₃⁻, NH₄⁺, PO₄³⁻), which root hairs then absorb through osmosis and active transport. In regions with low rainfall, poor solubility can limit nutrient availability—even if fertilizers are present. This illustrates how water acts not just as a passive carrier but as an active enabler of biological productivity.
Environmental and Industrial Applications
Outside biology, water’s solvent capabilities shape geological and industrial landscapes. Over millennia, slightly acidic rainwater (due to dissolved CO₂ forming carbonic acid) dissolves limestone bedrock, carving out caves and sinkholes in karst topography. This slow chemical weathering demonstrates how water facilitates large-scale Earth transformations.
In industry, water serves as a primary medium for chemical synthesis, cleaning, and extraction. Pharmaceutical manufacturing often uses aqueous solutions to purify compounds, while wastewater treatment plants depend on water’s ability to suspend and react with contaminants before removing them.
| Application | Role of Water as Solvent |
|---|---|
| Human Physiology | Transports oxygen, nutrients, and waste products in blood and cells |
| Agriculture | Dissolves soil minerals for plant uptake |
| Geology | Weathers rocks and transports dissolved minerals in rivers |
| Pharmaceuticals | Used in drug formulation and purification processes |
| Household Cleaning | Dissolves soaps and removes water-soluble stains |
Limits of Water’s Solvency: What It Can’t Dissolve
Despite its reputation, water cannot dissolve everything. Nonpolar substances such as oils, fats, waxes, and many organic solvents lack charged regions and therefore do not interact favorably with polar water molecules. Instead of dissolving, they tend to aggregate into separate phases—a phenomenon observed when oil floats on water.
This limitation is actually beneficial in biological systems. Cell membranes are made of phospholipid bilayers, where nonpolar tails face inward, shielded from water, while polar heads interact with the aqueous environment. This structural stability depends on water’s inability to dissolve hydrophobic components.
To dissolve nonpolar substances, chemists often turn to organic solvents like ethanol, acetone, or hexane. However, these lack the safety and environmental compatibility of water, underscoring why aqueous solutions remain preferred in biological and consumer applications.
Checklist: Maximizing Water’s Solvent Efficiency
- Ensure proper temperature control—warm water generally increases solubility of solids
- Agitate or stir solutions to speed up dissolution
- Break solutes into smaller particles to increase surface area
- Adjust pH when necessary to enhance ionization of weak acids or bases
- Avoid contamination with nonpolar residues that can inhibit mixing
Frequently Asked Questions
Why doesn’t water dissolve plastic?
Most plastics are composed of long-chain polymers with nonpolar covalent bonds. Since water is highly polar, it cannot overcome the strong intermolecular forces within these materials. As a result, plastics remain intact in water unless exposed to specific chemical treatments or extreme conditions over long periods.
Can water dissolve gases?
Yes, water can dissolve gases such as oxygen, carbon dioxide, and nitrogen. This is crucial for aquatic life—fish rely on dissolved oxygen in water for respiration. The amount of gas dissolved depends on temperature, pressure, and salinity. Cold water holds more dissolved gas than warm water.
Is distilled water a better solvent than tap water?
Distilled water is purer, containing fewer dissolved ions and impurities, which makes it more effective at dissolving certain substances without interference. However, in most practical cases, tap water performs similarly. In laboratory settings, distilled or deionized water is preferred to avoid unwanted chemical reactions.
Conclusion: Embracing Water’s Unique Role
Water’s designation as the universal solvent stems from its exceptional ability to dissolve a vast array of substances, particularly those critical to life. Its polar nature, hydrogen bonding, and high dielectric constant enable it to mediate chemical reactions, sustain biological functions, and shape the physical world. While it has limitations—especially with nonpolar compounds—its advantages far outweigh them in natural and human-designed systems.
Understanding this property deepens appreciation for everyday phenomena, from brewing coffee to the circulation of nutrients in ecosystems. Whether you're studying biology, managing agricultural resources, or simply curious about nature, recognizing water’s solvent power reveals the invisible chemistry underpinning life itself.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?