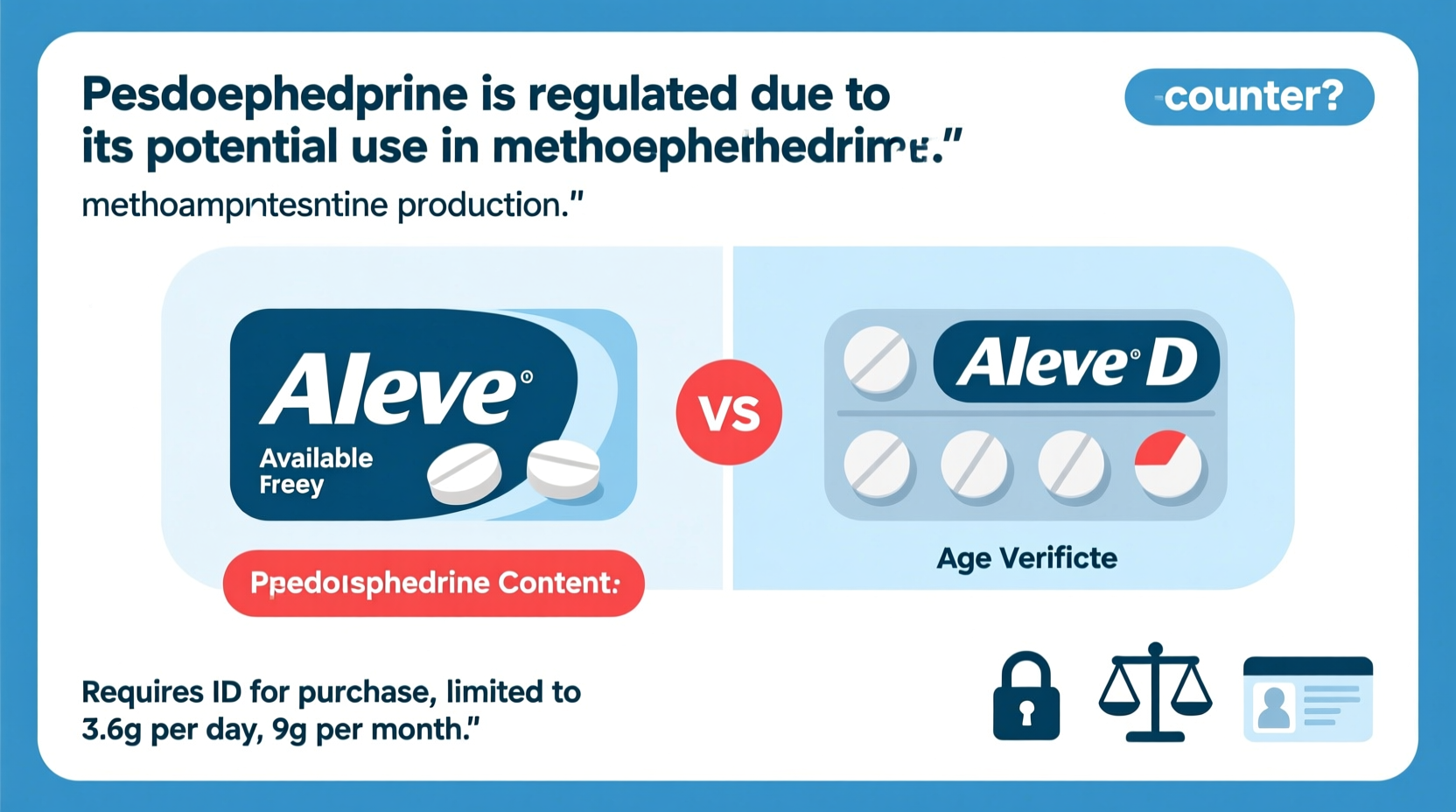
Aleve D, a common over-the-counter medication used to relieve sinus pressure and pain, isn’t found on regular pharmacy shelves like most cold and allergy remedies. Instead, it’s stored behind the counter, requiring customers to request it from a pharmacist. This restriction isn’t arbitrary—it stems directly from federal regulations surrounding one of its active ingredients: pseudoephedrine. Understanding the rationale behind these rules reveals a broader effort to balance public health needs with national safety concerns.
Pseudoephedrine is an effective decongestant that reduces nasal congestion by constricting blood vessels in the nasal passages. However, its chemical structure also makes it a key ingredient in the illegal production of methamphetamine. Over the past two decades, this dual-use nature has led to strict controls, reshaping how medications like Aleve D are sold across the United States.
The Link Between Pseudoephedrine and Methamphetamine Production
In the 1990s and early 2000s, law enforcement agencies observed a sharp rise in small-scale meth labs, often operating in homes, motels, or vehicles. These operations relied heavily on readily available cold medicines containing pseudoephedrine. The process—commonly known as \"cooking\"—converts pseudoephedrine into methamphetamine using relatively simple chemical methods. Because these medicines were once freely available, individuals could purchase large quantities without scrutiny, fueling a growing epidemic.
The ease of access became a national concern. According to the U.S. Drug Enforcement Administration (DEA), in 2004, more than 17,000 meth labs were dismantled nationwide. Many of these labs used pseudoephedrine extracted from over-the-counter products. The environmental and public health risks—from toxic fumes to chemical explosions—further amplified the urgency for regulatory action.
The Combat Methamphetamine Epidemic Act of 2005
In response, Congress passed the Combat Methamphetamine Epidemic Act (CMEA), which was signed into law in March 2006 as part of the larger USA PATRIOT Improvement and Reauthorization Act. This legislation fundamentally changed how pseudoephedrine-containing products are sold.
Key provisions of the CMEA include:
- Moving all products containing pseudoephedrine, ephedrine, or phenylpropanolamine to behind-the-counter status.
- Requiring photo ID for purchase.
- Mandating that retailers maintain a real-time log of purchases, either electronically or in bound paper logs.
- Limiting individual purchase amounts: up to 3.6 grams per day and 9 grams per 30-day period.
- Prohibiting sales to anyone under 18 years of age.
These restrictions apply uniformly across brands, including Aleve D, Sudafed, Claritin-D, and others. While consumers still have access to these medications, they must now interact with pharmacy staff and verify their identity before buying.
“Pseudoephedrine is one of the most critical precursor chemicals in illicit meth production. By controlling its retail distribution, we’ve significantly disrupted domestic meth manufacturing.” — DEA Special Agent in Charge, Midwest Region
Why Aleve D Specifically Is Affected
Aleve D combines naproxen sodium—a nonsteroidal anti-inflammatory drug (NSAID)—with pseudoephedrine hydrochloride. The latter is responsible for nasal decongestion but also triggers regulatory oversight. Even though Aleve D is marketed primarily for sinus pain and congestion relief, its pseudoephedrine content places it under the same legal framework as other cold medicines containing the compound.
Unlike regular Aleve (which contains only naproxen and remains on open shelves), Aleve D cannot be self-served. Pharmacies must store it securely and track every transaction through systems like NPLEx (Narcotics Precursor Log Exchange), a national electronic tracking network that alerts retailers when a customer exceeds legal purchase limits.
Impact on Consumers and Public Health
The regulations have had measurable effects. According to a 2019 study published in the American Journal of Public Health, states that implemented strict pseudoephedrine sales tracking saw a 40–60% reduction in meth lab incidents within five years. The national availability of domestically produced meth also declined, although trafficked meth from international sources has since increased—a complex shift that continues to challenge policymakers.
For legitimate users, the inconvenience is real. Patients with chronic sinus conditions or seasonal allergies may find the purchase process cumbersome. Some opt for alternative decongestants like phenylephrine, but studies suggest it is less effective than pseudoephedrine. Others turn to prescription options, which can involve additional costs and doctor visits.
Pharmacies play a gatekeeping role, ensuring compliance while assisting patients. Pharmacists are trained to recognize suspicious behavior, such as frequent large purchases or attempts to buy under different names. They also educate consumers about alternatives and proper use.
Do’s and Don’ts When Buying Aleve D
| Do’s | Don’ts |
|---|---|
| Bring a government-issued photo ID (driver’s license, passport) | Attempt to purchase under someone else’s name |
| Know your monthly limit (9 grams of pseudoephedrine) | Buy from multiple pharmacies on the same day |
| Ask your pharmacist about alternative treatments if restricted | Hoarding product “just in case” |
| Use the NPLEx app to track your own purchases | Assume online purchases are unregulated—they’re not; reputable sites follow the same rules |
Real-World Scenario: A Chronic Sinus Sufferer’s Experience
Consider Sarah, a 42-year-old teacher with year-round allergies. Every fall and spring, she relies on Aleve D to manage congestion and facial pain. After moving to a new city, she visited three different pharmacies trying to stock up before allergy season. At the third location, she was denied purchase. Confused and frustrated, she spoke with the pharmacist, who explained that the national tracking system flagged her as exceeding the 9-gram monthly limit.
Though inconvenient, the system worked as intended. Sarah learned she could plan better by spacing out purchases or discussing longer-term solutions with her allergist. She later switched to a prescription nasal spray, reducing her reliance on pseudoephedrine altogether. Her experience highlights both the friction and the value of the current regulatory model.
Alternatives and Future Trends
As regulations remain firm, pharmaceutical companies have responded by reformulating products. Many now offer “D-free” versions or switch to phenylephrine, though consumer feedback often criticizes its weaker efficacy. Researchers continue exploring new decongestants that avoid precursor status while maintaining effectiveness.
Some states, like Oregon and Mississippi, have gone further by requiring a prescription for all pseudoephedrine products. While this reduces diversion, it also creates access barriers for rural or low-income patients. The debate continues between public safety and patient autonomy.
Step-by-Step Guide: How to Purchase Aleve D Legally and Smoothly
- Check your local pharmacy’s policy: Some stores require you to ask at the service desk; others have dedicated counters.
- Bring valid photo ID: This is mandatory regardless of age.
- Be honest about your intended use: Pharmacists may ask; transparency builds trust.
- Know your purchase history: Use the NPLEx website or app to track your recent buys.
- Consider alternatives if blocked: Ask about non-pseudoephedrine options or consult your doctor.
Frequently Asked Questions
Can I buy Aleve D online?
Yes, but the same federal rules apply. Reputable online pharmacies will verify your identity, require a mailing address (no P.O. boxes), and log your purchase in the NPLEx system. Quantity limits still apply.
Why is pseudoephedrine still available if it’s used for meth?
Because it remains one of the most effective oral decongestants. Banning it entirely would deprive millions of safe, lawful users of a valuable treatment. Regulated access strikes a balance between medical utility and abuse prevention.
Are there any exceptions to the ID requirement?
No. Federal law requires photo ID for every purchase. There are no exemptions—even for repeat customers or healthcare workers.
Conclusion
The reason Aleve D is kept behind the counter lies in a deliberate, long-standing effort to curb the misuse of pseudoephedrine in meth production. While the process may seem inconvenient, it reflects a national compromise: preserving access to effective medicine while protecting communities from the dangers of illegal drug manufacturing. These regulations have demonstrably reduced domestic meth lab incidents and forced traffickers to rely on more complex supply chains.
As a consumer, understanding the “why” behind the rule empowers smarter choices. Whether you're managing seasonal allergies or supporting someone who uses these medications regularly, awareness of the system helps ensure smooth access and informed decisions.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?