ISO 9001 is the internationally recognized standard for Quality Management Systems (QMS), used by organizations across industries to demonstrate their ability to consistently provide products and services that meet customer and regulatory requirements. In the United States, achieving ISO 9001 certification can open doors to new markets, improve operational efficiency, and enhance credibility with clients and stakeholders. However, the path to certification can seem complex without clear direction. This guide breaks down the process into manageable steps, offering actionable insights and real-world strategies to help U.S.-based organizations achieve compliance efficiently.
Understanding ISO 9001: What It Means for Your Organization
ISO 9001 is not a one-size-fits-all regulation but a framework built on seven quality management principles, including customer focus, leadership, engagement of people, process approach, improvement, evidence-based decision making, and relationship management. Certification indicates that your organization has implemented a documented QMS that meets these standards and is capable of delivering consistent quality.
In the U.S., ISO 9001 is particularly valuable for companies in manufacturing, healthcare, technology, and service sectors. While it's not legally required, many federal contracts, suppliers, and international partners expect or require certification as proof of reliability and process maturity.
“ISO 9001 isn’t about perfection—it’s about continuous improvement through structured processes.” — Dr. Linda Peterson, Quality Systems Consultant, ASQ Fellow
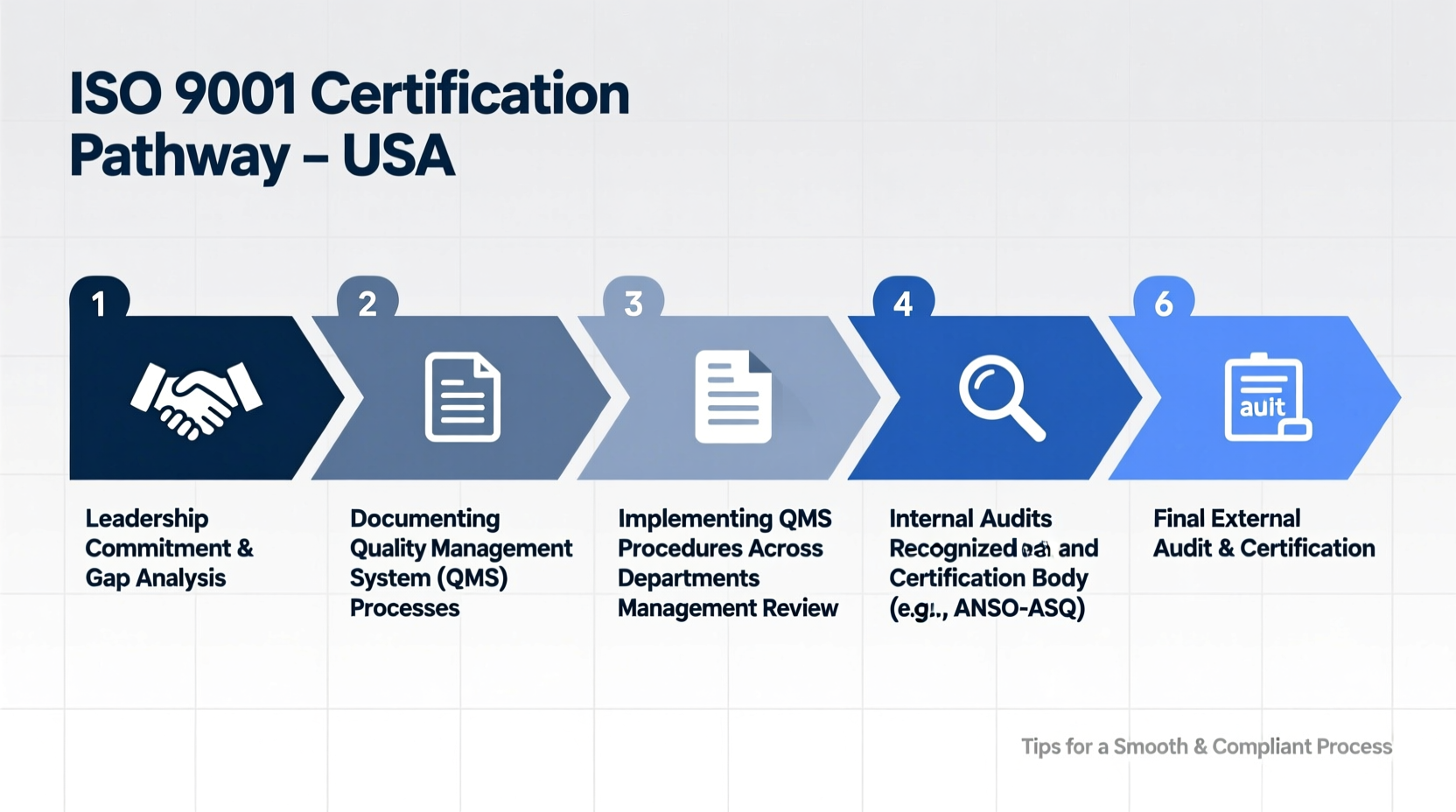
Step-by-Step Timeline to ISO 9001 Certification
Achieving certification typically takes between 6 to 18 months, depending on the size and complexity of your organization. Below is a realistic timeline broken into key phases:
- Gap Analysis (Month 1–2): Assess current processes against ISO 9001:2015 requirements.
- Planning & Leadership Buy-In (Month 2–3): Secure executive support and define scope, objectives, and resources.
- Documentation Development (Month 3–5): Create or update quality manuals, procedures, work instructions, and records.
- Implementation & Training (Month 5–7): Roll out the QMS across departments and train employees.
- Internal Audit (Month 7–8): Conduct audits to verify compliance and identify non-conformities.
- Management Review (Month 8): Evaluate system performance and readiness for external audit.
- Stage 1 Audit (Month 9): Initial review by a third-party certification body.
- Stage 2 Audit (Month 10): Full assessment of implementation and effectiveness.
- Certification Issuance (Month 11): Receive certificate upon successful audit completion.
- Surveillance Audits (Ongoing): Annual reviews to maintain certification.
Essential Components of an Effective QMS Implementation
To pass the certification audit, your QMS must demonstrate not just documentation, but actual integration into daily operations. Key components include:
- Context of the Organization: Identify internal and external issues affecting quality (e.g., supply chain risks, regulatory changes).
- Interested Parties: Map customers, regulators, employees, and suppliers and their expectations.
- Risk-Based Thinking: Proactively address risks and opportunities in all processes.
- Documented Information: Maintain control over documents and records per Clause 7.5.
- Operational Control: Define processes for design, production, service delivery, and supplier management.
- Performance Evaluation: Monitor KPIs, conduct internal audits, and hold management reviews.
- Improvement: Establish corrective action procedures and continual improvement mechanisms.
Checklist: Preparing for Stage 1 Audit
- Completed gap analysis report
- Approved quality policy and objectives
- Defined scope of the QMS
- Updated organizational chart with roles and responsibilities
- Documented procedures for key processes (e.g., document control, internal audit)
- Records of employee training related to QMS
- Scheduled internal audit and management review meetings
- Selection of accredited certification body
Avoiding Common Pitfalls: Do’s and Don’ts
Many organizations fail their first audit due to preventable errors. The table below outlines frequent missteps and how to avoid them.
| Do’s | Don’ts |
|---|---|
| Engage leadership from day one | Leave ISO efforts solely to the quality department |
| Train all employees on QMS basics | Assume only managers need to understand ISO 9001 |
| Keep documentation simple and practical | Create overly complex manuals that no one uses |
| Conduct regular internal audits | Wait until the audit to fix known issues |
| Use risk-based thinking proactively | Treat risk assessment as a paperwork exercise |
| Maintain accurate, accessible records | Store documents in personal folders or uncontrolled formats |
Real-World Example: How a Mid-Sized Manufacturer Achieved Certification
A precision machining company in Ohio with 75 employees decided to pursue ISO 9001 certification to qualify for aerospace contracts. Initially, they underestimated the cultural shift required. Their first gap analysis revealed inconsistent documentation, lack of defined processes, and minimal employee awareness.
The leadership team appointed a full-time ISO coordinator, conducted workshops across departments, and integrated QMS tasks into existing workflows. They simplified procedures using flowcharts and digital checklists. After six months of preparation, they passed both Stage 1 and Stage 2 audits with only two minor non-conformities related to calibration records.
Within a year of certification, they secured three new government contracts and reduced customer complaint resolution time by 40%. More importantly, employees reported clearer job expectations and better interdepartmental communication.
“We thought ISO was just about paperwork. It turned out to be a tool for real operational clarity.” — Mark Reynolds, Operations Director, Apex Machining Inc.
Frequently Asked Questions
How much does ISO 9001 certification cost in the USA?
Costs vary based on company size and complexity. For small businesses (10–50 employees), total expenses typically range from $5,000 to $15,000, covering consulting, training, documentation tools, and audit fees. Larger organizations may spend $25,000 or more. Accredited certification bodies usually charge $3,000–$7,000 for the audit itself.
Can I get ISO 9001 certified without hiring a consultant?
Yes, many small to mid-sized businesses self-implement using free resources from ANSI (American National Standards Institute) and NIST (National Institute of Standards and Technology). However, consultants can accelerate the process, especially if internal staff lack experience with management systems. The key is having someone internally who understands the standard and can drive implementation.
How long is ISO 9001 certification valid?
Certification is valid for three years, provided you pass annual surveillance audits. These audits ensure ongoing compliance and effective continual improvement. At the end of the three-year cycle, a re-certification audit is required.
Final Steps and Moving Forward
Once certified, the work doesn’t stop. ISO 9001 is not a destination but a commitment to ongoing quality improvement. Successful organizations treat the QMS as a living system—reviewing performance data, updating processes, and empowering employees to suggest improvements.
Maintaining certification requires discipline, but the benefits are lasting: improved customer satisfaction, streamlined operations, stronger supplier relationships, and increased competitiveness. Whether you're aiming for federal contracts, global expansion, or internal excellence, ISO 9001 provides a proven roadmap.









 浙公网安备
33010002000092号
浙公网安备
33010002000092号 浙B2-20120091-4
浙B2-20120091-4
Comments
No comments yet. Why don't you start the discussion?